Moderna COVID-19 vaccine (redirect from MRNA-1273)
mRNA-1273/background/2021.1. Scholia has a profile for mRNA-1273 vaccine (Q87775025). Wikimedia Commons has media related to MRNA-1273. Wikinews...
177 KB (13,698 words) - 16:52, 23 October 2024
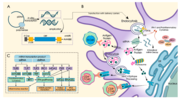
An mRNA vaccine is a type of vaccine that uses a copy of a molecule called messenger RNA (mRNA) to produce an immune response. The vaccine delivers molecules...
77 KB (7,946 words) - 04:47, 22 December 2024
Lipid-based nanoparticle (redirect from MRNA-lipid nanoparticle)
a complex of plasmid or linear DNA and lipids Targeted drug delivery mRNA-1273, from Moderna, uses LNPs BNT162b2, from BioNTech/Pfizer, uses LNPs Mehta...
26 KB (2,937 words) - 04:37, 9 December 2024
COVID-19 vaccine (redirect from MRNA-lipid nanoparticle COVID-19 vaccines)
mRNA-1273/background/2021.1. Archived from the original on 13 June 2021. Retrieved 24 July 2021. "Background document on the mRNA-1273 vaccine...
207 KB (23,493 words) - 04:04, 30 December 2024
the Moderna COVID‑19 vaccine candidate, and in December, the vaccine, mRNA-1273, was issued an emergency use authorization in the United States. In 2022...
27 KB (2,177 words) - 01:05, 25 December 2024
PMID 30785039. Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19), clinicaltrials.gov...
7 KB (628 words) - 18:17, 27 March 2024
Nucleoside-modified messenger RNA (redirect from Nucleoside-modified mRNA)
BioNTech/Pfizer (BNT162b2), and by Moderna (mRNA-1273). The zorecimeran vaccine developed by Curevac, however, uses unmodified mRNA, instead relying on codon optimization...
24 KB (2,503 words) - 15:45, 11 November 2024
which biotechnology company Moderna used as the basis for the vaccine mRNA-1273, the first COVID-19 vaccine candidate to enter phase I clinical trials...
30 KB (2,830 words) - 18:33, 2 October 2024
Pfizer–BioNTech COVID‑19 vaccine. A week later, they granted an EUA for mRNA-1273 (active ingredient elasomeran), the Moderna vaccine. On 31 March 2021...
122 KB (11,033 words) - 06:12, 17 November 2024
Study to Evaluate Safety, Reactogenicity, and Immunogenicity of mRNA-1283 and mRNA-1273 Vaccines in Healthy Adults Between 18 Years and 55 Years of Age...
5 KB (387 words) - 20:39, 30 May 2024
2020, Moderna struck a manufacturing deal with Lonza to produce its (mRNA-1273) COVID-19 vaccine active ingredient while expanding sterile production...
19 KB (1,866 words) - 08:05, 3 December 2024
; Hernán, Miguel A. (2022-07-01). "Comparative Safety of BNT162b2 and mRNA-1273 Vaccines in a Nationwide Cohort of US Veterans". JAMA Internal Medicine...
16 KB (1,511 words) - 13:39, 11 December 2024
PMC 8491089. PMID 34326008. S2CID 236515442. Background document on the mRNA-1273 vaccine (Moderna) against COVID-19 (Report). World Health Organization...
288 KB (33,194 words) - 15:09, 26 December 2024
interim results showing 86% efficacy. While mRNA vaccines like the Pfizer–BioNTech COVID-19 vaccine and mRNA-1273 showed higher efficacy of over 90%, those...
17 KB (1,214 words) - 18:37, 20 October 2024
Buchan SA, Wilson SE, et al. (August 2021). "Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe...
260 KB (22,123 words) - 05:02, 30 December 2024
via public health clinics. The province began to administer the Moderna MRNA-1273 vaccine in early-January 2021, with an initial focus on Northern Saskatchewan...
257 KB (22,394 words) - 07:24, 25 June 2024
PEGylated lipid nanoparticle drug delivery (LNP) system of the mRNA vaccine known as mRNA-1273 has been the subject of ongoing patent litigation with Arbutus...
27 KB (2,986 words) - 00:38, 24 October 2024
vaccines: AZD1222 from Oxford University and AstraZeneca on 30 December, mRNA-1273 from Moderna on 8 January 2021, and a single-dose vaccine from Janssen...
26 KB (2,421 words) - 18:24, 19 November 2024
and vote on regulations relating to public health. On 8 January 2021, MRNA-1273 (commonly known as the Moderna COVID-19 vaccine) was the third COVID-19...
181 KB (19,466 words) - 20:44, 12 September 2024
vaccines approved for use in humans include the RNA vaccines tozinameran and mRNA-1273, the DNA vaccine ZyCoV-D as well as the viral vectors AZD1222, Ad26.COV2...
8 KB (1,186 words) - 05:09, 11 June 2024
et al. (29 September 2020). "Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults". The New England Journal of Medicine. doi:10...
12 KB (1,228 words) - 17:37, 25 August 2024
Benslimane FM, Al Khatib HA, Tang P, Hasan MR, et al. (September 2021). "mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants...
19 KB (1,608 words) - 14:09, 7 June 2024
advisory panel to consider recommending emergency use authorization of the mRNA-1273 vaccine. Moore is married to Janet Kosloff, a retired CEO and entrepreneur...
11 KB (895 words) - 22:17, 24 September 2024
January 2021. "Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19)". ClinicalTrials.gov...
322 KB (19,558 words) - 03:15, 23 November 2024
Other recent applications include two FDA approved COVID-19 vaccines: mRNA-1273, developed by Moderna. and BNT162b, developed by a collaboration between...
140 KB (15,409 words) - 20:24, 5 December 2024
A. Doria-Rose; Xiaoying Shen; et al. (20 December 2021). "Booster of mRNA-1273 Strengthens SARS-CoV-2 Omicron Neutralization". medRxiv 10.1101/2021.12...
299 KB (38,958 words) - 21:53, 29 December 2024
Melanie; Miller, Jacqueline; Zaks, Tal (2021). "Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine". New England Journal of Medicine. 384 (5): 403–416...
5 KB (462 words) - 16:13, 4 May 2023
for the original strain. Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) mRNA vaccines provide reduced protection against asymptomatic disease but...
219 KB (24,993 words) - 15:39, 24 December 2024
of Protection Against Symptomatic COVID-19 Among Participants of the mRNA-1273 SARS-CoV-2 Vaccine Trial. JAMA Network Open 5: e2215984 Lin DY, Gu Y,...
18 KB (1,665 words) - 04:39, 11 November 2024
vaccine. In the U.S., the VRBPAC supported an EUA for Moderna's mRNA vaccine, mRNA-1273. World Health Organization (WHO) European Medicines Agency (EMA)...
12 KB (1,380 words) - 17:54, 24 February 2024