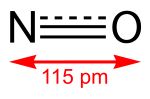
Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary...
17 KB (2,019 words) - 15:22, 2 September 2024
form homoleptic and mixed ligand complexes that are analogous to the metal carbonyls.[citation needed] Metal nitrosyls, compounds featuring NO ligands...
70 KB (7,931 words) - 06:04, 29 May 2024
turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that...
57 KB (5,553 words) - 04:29, 2 October 2024
nitrito complexes by base-hydrolysis of electrophilic metal nitrosyls. Nitro complexes also catalyze the oxidation of alkenes. Metal nitrito complexes figure...
9 KB (1,050 words) - 15:50, 30 May 2024
In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia (NH3) ligand. "Ammine" is spelled this way for historical...
16 KB (1,787 words) - 12:15, 19 July 2024
Nitrosonium (section As a source of nitrosyl complexes)
NOBF4 reacts with some metal carbonyl complexes to yield related metal nitrosyl complexes. In some cases, [NO]+ does not bind the metal nucleophile but acts...
6 KB (615 words) - 23:46, 31 August 2024
chloride is used to prepare metal nitrosyl complexes. With molybdenum hexacarbonyl, NOCl gives the dinitrosyldichloride complex: Mo(CO)6 + 2 NOCl → MoCl2(NO)2...
10 KB (957 words) - 21:33, 31 August 2024
Nitric oxide (redirect from Nitrosyl radical)
N-methoxydiazeniumdiolate. Nitric oxide reacts with transition metals to give complexes called metal nitrosyls. The most common bonding mode of nitric oxide is the...
31 KB (2,970 words) - 16:06, 29 October 2024
Metal nitrido complexes are coordination compounds and metal clusters that contain an atom of nitrogen bound only to transition metals. These compounds...
8 KB (808 words) - 05:32, 22 June 2024
The related pentamethylcyclopentadienyl complex (C5(CH3)5)NiNO is also known. Cyclopentadienyl nickel nitrosyl was the subject of several patents as a...
5 KB (316 words) - 08:58, 11 July 2024
process as adding a nitrosyl radical NO•. Nitrosylation commonly occurs in the context of a metal (e.g. iron) or a thiol, leading to nitrosyl iron Fe−NO (e...
8 KB (894 words) - 19:59, 21 October 2024
Nitrite (section Coordination complexes)
wide variety of coordination complexes by binding to metal ions in several ways. Two examples are the red nitrito complex [Co(NH3)5(ONO)]2+ is metastable...
26 KB (2,641 words) - 22:18, 29 October 2024
PETN—"around 50 times less than was used—would be enough to blast a hole in a metal plate twice the thickness of an aircraft's skin". In contrast, according...
35 KB (3,471 words) - 01:05, 17 October 2024
Nitrogen compounds (section Dinitrogen complexes)
dioxide and with halogens to give nitrosyl halides. It also reacts with transition metal compounds to give nitrosyl complexes, most of which are deeply coloured...
34 KB (4,298 words) - 07:31, 17 May 2024
from the formation of nitrosyl chloride and nitrogen dioxide. It was so named by alchemists because it can dissolve noble metals like gold and platinum...
17 KB (1,841 words) - 05:12, 18 October 2024
redox reaction of nitrosonium and the metal can give rise to nitrogen oxide which forms strong metal nitrosyl complexes; nitronium ions (NO2+) are similarly...
11 KB (1,122 words) - 22:26, 27 August 2024
the reaction of nitrous acid (HNO2) and secondary amines, although other nitrosyl sources (e.g. N 2O 4, NOCl, RONO) have the same effect: HONO + R2NH → R2N-NO...
11 KB (1,851 words) - 15:41, 20 September 2024
classes of protein. Nitric oxide to a transition metal ion like iron or copper, forming metal nitrosyl complexes. Typical cases involve the nitrosylation of...
70 KB (7,668 words) - 07:22, 6 August 2024
Sodium nitroprusside (category Nitrosyl complexes)
(2002). "Photoinduced Linkage Isomers of Transition-Metal Nitrosyl Compounds and Related Complexes". Chemical Reviews. 102 (4): 861–883. doi:10.1021/cr000031c...
40 KB (4,132 words) - 21:09, 15 March 2024
can be used to oxidize several transition metal and main group element complexes. The first metal nitrosyl cation was prepared using the PFAA's [Al(Opftb)4]−...
29 KB (2,984 words) - 02:37, 21 August 2023
the simplest metal nitrosyls. It is highly toxic, reminiscent of the same property for nickel tetracarbonyl. Cobalt tricarbonyl nitrosyl is prepared by...
2 KB (155 words) - 19:52, 18 October 2023
thiolate ligands are cysteinyl residues or glutathione. These metal nitrosyl complexes have attracted much attention because they serve as biochemical...
1 KB (150 words) - 19:53, 3 September 2023
SNO-vWF; N-Nitroso compounds (e.g., nitrosamines): SIN-1A Nitrosyl compounds: Metal nitrosyl complexes: Roussin's black salt Roussin's red salt Sodium nitroprusside...
19 KB (1,859 words) - 21:53, 9 October 2024
SNO-vWF; N-Nitroso compounds (e.g., nitrosamines): SIN-1A Nitrosyl compounds: Metal nitrosyl complexes: Roussin's black salt Roussin's red salt Sodium nitroprusside...
40 KB (4,209 words) - 14:12, 29 October 2024
Transition metal sulfate complexes or sulfato complexes are coordination complexes with one or more sulfate ligands. Sulfate binds to metals through one...
7 KB (649 words) - 18:14, 3 April 2024
an even stronger π-acceptor than CO and νNO is a diagnostic tool in metal–nitrosyl chemistry. Isocyanides, RNC, are another class of ligands that are capable...
11 KB (1,291 words) - 04:15, 18 October 2024
blue) graphite hydrogen sulfate C+ 24HSO– 4 • 2.4H2SO4. Nitrogen forms nitrosyl hydrogen sulfate (NO)HSO4 and nitronium (or nitryl) hydrogen sulfate (NO2)HSO4...
66 KB (9,098 words) - 18:15, 30 October 2024
principally operates as a Ca2+ binding protein, it also coordinates other metal ions. For example, in the presence of typical intracellular concentrations...
37 KB (4,221 words) - 08:11, 28 August 2024
SNO-vWF; N-Nitroso compounds (e.g., nitrosamines): SIN-1A Nitrosyl compounds: Metal nitrosyl complexes: Roussin's black salt Roussin's red salt Sodium nitroprusside...
3 KB (66 words) - 14:05, 15 August 2024