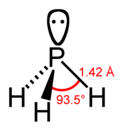
In chemistry, the term phosphonium (more obscurely: phosphinium) describes polyatomic cations with the chemical formula PR+ 4 (where R is a hydrogen or...
13 KB (1,231 words) - 23:45, 1 June 2023
Wittig reaction (redirect from Phosphonium ylide)
olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used...
15 KB (1,600 words) - 07:36, 18 October 2024
Ylide (section Phosphonium ylides)
Consequently, the carbon anion is trigonal pyramidal.[citation needed] Phosphonium ylides are used in the Wittig reaction, a method used to convert ketones...
10 KB (1,152 words) - 18:33, 21 August 2024
In organic chemistry, phosphonium coupling is a cross-coupling reaction for organic synthesis. It is a mild, efficient, chemoselective and versatile methodology...
3 KB (429 words) - 00:36, 26 August 2022
Wittig reagents (section Formation of phosphonium salt)
to be sold commercially. Wittig reagents are usually prepared from a phosphonium salt, which is in turn prepared by the quaternization of triphenylphosphine...
7 KB (714 words) - 02:26, 19 June 2024
dialkylthiophosphinate ester.: 55 Compounds with the formula [PR4+]X− comprise the phosphonium salts. These species are tetrahedral phosphorus(V) compounds. From the...
18 KB (1,968 words) - 03:41, 23 December 2024
Phosphonium iodide is a chemical compound with the formula PH 4I. It is an example of a salt containing an unsubstituted phosphonium cation (PH+ 4). Phosphonium...
6 KB (540 words) - 02:39, 23 October 2024
group that bears no hydrogen atom, such as a quaternary ammonium or phosphonium cation (generally: onium ions), and with a negatively charged functional...
6 KB (619 words) - 14:21, 9 June 2024
(1 - A phosphite) with the electrophilic alkyl halide (2) to give a phosphonium salt as an intermediate (3). These intermediates are occasionally stable...
13 KB (1,591 words) - 16:54, 5 November 2024
intermediate formed when using a carbodiimide reagent, an amidinium- or phosphonium-reagent can be employed These reagents have two parts: an electrophilic...
55 KB (6,166 words) - 21:04, 15 December 2024
Decyl(triphenyl)phosphonium (DTPP) is the organophosphorus cation with the formula C10H21P(C6H5)3+. It is a lipophilic quaternary phosphonium cation. It forms...
4 KB (252 words) - 15:50, 18 October 2024
synthesis of alkenes from aldehydes and ketones using compounds called phosphonium ylides in the Wittig reaction. He shared the Nobel Prize in Chemistry...
7 KB (730 words) - 03:39, 1 January 2025
Tetrakis(hydroxymethyl)phosphonium chloride (THPC) is an organophosphorus compound with the chemical formula [P(CH2OH)4]Cl. It is a white water-soluble...
7 KB (709 words) - 00:20, 9 December 2024
by analogy with the corresponding phosphonium salts, which bear a positive carbon atom instead of the phosphonium residue. Later, it was shown by X-ray...
8 KB (639 words) - 12:03, 1 August 2024
Phosphonate Phosphite Phosphonous Phosphinate Phosphine oxide Phosphine Phosphonium Phosphaalkene Phosphaalkyne Phosphaallene Sulfur Thiol Sulfide Sulfonium...
91 KB (9,589 words) - 07:53, 16 December 2024
but acid and base activity is poor. Proton exchange proceeds via a phosphonium (PH+4) ion in acidic solutions and via phosphanide (PH−2) at high pH...
39 KB (3,618 words) - 00:41, 28 December 2024
Bis(triphenylphosphine)iminium chloride is the chloride salt of a bulky lipophilic phosphonium cation [Ph3PNPPh3]+. Tetraphenylphosphonium chloride (C6H5)4PCl, abbreviated...
6 KB (692 words) - 19:26, 27 October 2022
Phosphonate Phosphite Phosphonous Phosphinate Phosphine oxide Phosphine Phosphonium Phosphaalkene Phosphaalkyne Phosphaallene Sulfur Thiol Sulfide Sulfonium...
26 KB (2,619 words) - 12:07, 27 December 2024
PyAOP reagent (category Quaternary phosphonium compounds)
reagent PyBOP Mansour, Tarek S.; Bardhan, Sujata; Wan, Zhao-Kui (2010). "Phosphonium- and Benzotriazolyloxy-Mediated Bond-Forming Reactions and Their Synthetic...
5 KB (350 words) - 16:17, 3 August 2024
base, e.g., for the deprotonation of ketones to form sodium enolates, phosphonium salts to form Wittig reagents, and formamidinium salts to form diaminocarbenes...
39 KB (4,029 words) - 23:40, 5 December 2024
methyltributylammonium chloride. Organic phosphonium salts are also used, e.g., hexadecyltributylphosphonium bromide. The phosphonium salts tolerate higher temperatures...
14 KB (1,747 words) - 16:06, 4 November 2024
the acid and base. Phosphonium cations (R4P+) are less common but offer some advantageous properties. Some examples of phosphonium cations are...
43 KB (4,706 words) - 07:07, 9 August 2024
located on a quaternary ammonium group. Similarly, a molecule containing a phosphonium group and a carboxylate group cannot isomerize. Tautomerism of amino...
11 KB (1,106 words) - 01:24, 27 December 2024
Phosphonate Phosphite Phosphonous Phosphinate Phosphine oxide Phosphine Phosphonium Phosphaalkene Phosphaalkyne Phosphaallene Sulfur Thiol Sulfide Sulfonium...
64 KB (6,661 words) - 19:30, 4 December 2024
Phosphonate Phosphite Phosphonous Phosphinate Phosphine oxide Phosphine Phosphonium Phosphaalkene Phosphaalkyne Phosphaallene Sulfur Thiol Sulfide Sulfonium...
19 KB (1,843 words) - 18:54, 16 December 2024
Kohlenwasserstoffe durch Jodphosphonium" [On the reduction of aromatic compound by phosphonium iodide [H4IP]]. Annalen der Chemie und Pharmacie. 55: 266–281. Bei der...
18 KB (1,603 words) - 22:59, 16 December 2024
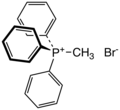
Methyltriphenylphosphonium bromide (category Quaternary phosphonium compounds)
compound with the formula [(C6H5)3PCH3]Br. It is the bromide salt of a phosphonium cation. It is a white salt that is soluble in polar organic solvents...
2 KB (119 words) - 22:43, 27 May 2023
tertiary phosphines are treated with alkyl halides, the products being phosphonium salts. Thiols are readily alkylated to give thioethers via the thiol-ene...
15 KB (1,651 words) - 13:59, 24 August 2024
reaction with an electrophile gives a resonance stabilized cation With phosphonium ylides in the Wittig reaction to give the alkenes With thiols to give...
24 KB (2,895 words) - 03:58, 25 November 2024
triphenylphosphine dichloride ([PPh3Cl]Cl), which exists as the moisture-sensitive phosphonium halide. This reagent is used to convert alcohols to alkyl chlorides in...
18 KB (1,624 words) - 16:57, 12 June 2024