a halogen bond (XB or HaB) occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom...
25 KB (2,760 words) - 18:09, 20 August 2024
Haloalkane (redirect from Carbon-halogen bond)
as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although...
20 KB (2,413 words) - 06:14, 8 September 2024
The halogens (/ˈhælədʒən, ˈheɪ-, -loʊ-, -ˌdʒɛn/) are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine...
52 KB (5,499 words) - 17:27, 19 November 2024
Non-covalent interaction (redirect from Non-covalent bond)
similar to the dipole–dipole interaction known as hydrogen bonding. In halogen bonding, a halogen atom acts as an electrophile, or electron-seeking species...
27 KB (3,311 words) - 07:44, 12 November 2024
A halogen addition reaction is a simple organic reaction where a halogen molecule is added to the carbon–carbon double bond of an alkene functional group...
7 KB (801 words) - 18:25, 20 April 2021
Molecular solid (section Hydrogen and halogen bonding)
interactions, quadrupole interactions, π–π interactions, hydrogen bonding, halogen bonding, London dispersion forces, and in some molecular solids, coulombic...
33 KB (2,941 words) - 16:03, 25 August 2024
respect to their heavier analogues. In some cases a similar halogen bond can be formed by a halogen atom located between two electronegative atoms on different...
40 KB (4,872 words) - 13:33, 22 September 2024
In chemistry, a chalcogen bond (ChB) is an attractive interaction in the family of σ-hole interactions, along with halogen bonds. Electrostatic, charge-transfer...
16 KB (1,988 words) - 11:12, 10 April 2024
also has an interest in crystal engineering, in particular by using the halogen bond. He is Vice-President and President-Elect of the Physical and Biophysical...
7 KB (589 words) - 17:09, 28 October 2024
states. The C–I bond is the weakest of the carbon–halogen bonds. These bond strengths correlate with the electronegativity of the halogen, decreasing in...
12 KB (1,416 words) - 23:42, 23 October 2024
can have a bond dissociation energy (BDE) of up to 130 kcal/mol. The BDE (strength of the bond) of C–F is higher than other carbon–halogen and carbon–hydrogen...
15 KB (1,701 words) - 19:03, 21 September 2024
Sigma hole interactions (category Chemical bonding)
tetrel bonding (in which a sigma hole resides on an atom of group IV), pnictogen bonding (group V), chalcogen bonding (group VI), and halogen bonding (group...
19 KB (2,170 words) - 14:49, 2 November 2024
Functional group (section Groups containing halogen)
Haloalkanes are a class of molecule that is defined by a carbon–halogen bond. This bond can be relatively weak (in the case of an iodoalkane) or quite...
32 KB (1,230 words) - 12:24, 22 October 2024
examples are molecules with a nitrogen-halogen bond, azo compounds, and organic and inorganic peroxides. Halogens undergo homolytic fission relatively easily...
3 KB (419 words) - 00:25, 3 October 2022
Iodine (category Halogens)
element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid...
104 KB (11,657 words) - 19:32, 24 November 2024
Chlorine (category Halogens)
element; it has symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its...
117 KB (13,065 words) - 23:27, 9 November 2024
metal into the carbon-halogen bond. However, such reactions are temperamental, typically requiring a very weak carbon-halogen bond (e.g. an alkyl iodide...
24 KB (2,623 words) - 18:21, 19 September 2024
shown to coordinate to Lewis acids through electrostatic, σ-hole (see halogen bond) interactions, between the Lewis basic oxygen atoms of the crown ether...
13 KB (1,460 words) - 00:03, 11 October 2024
one-step mechanism in which carbon-hydrogen and carbon-halogen bonds break to form a double bond (C=C Pi bond). The specifics of the reaction are as follows:...
14 KB (1,844 words) - 22:24, 27 May 2024
haloalkanes. If the two carbon atoms at the double bond are linked to a different number of hydrogen atoms, the halogen is found preferentially at the carbon with...
6 KB (788 words) - 07:43, 16 November 2023
Organobromine chemistry (redirect from Carbon-bromine bond)
carbon in a carbon–bromine bond is electrophilic, i.e. alkyl bromides are alkylating agents. Carbon–halogen bond strengths, or bond dissociation energies are...
13 KB (1,557 words) - 01:29, 22 March 2024
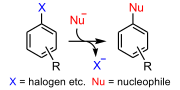
halides cannot undergo the classic 'backside' SN2 reaction. The carbon-halogen bond is in the plane of the ring because the carbon atom has a trigonal planar...
11 KB (1,295 words) - 12:42, 28 August 2024
low boiling points (−72 °C and −6 °C respectively) and short nitrogen–halogen bond lengths (N–F 135 pm, N–Cl 184 pm). Addition of one electron forms the...
5 KB (475 words) - 08:28, 1 November 2024
Polyhalogen ions (category Halogens)
a reduced bond order, all three halogen atoms are tightly bound. The fluorine–fluorine bond of trifluoride, with bond order 0.5, has a bond-strength is...
25 KB (2,037 words) - 22:22, 8 November 2024
reactions of the halogens). Chemical compounds with double bonds Ethylene Carbon-carbon double bond Acetone Carbon-oxygen double bond Dimethyl sulfoxide...
8 KB (933 words) - 06:40, 19 May 2024
A sextuple bond is a type of covalent bond involving 12 bonding electrons and in which the bond order is 6. The only known molecules with true sextuple...
18 KB (2,002 words) - 23:36, 20 October 2024
carbon-halogen bond through the formation of an epoxide from a vicinal hydroxyl group. The substrate binds to the active site through hydrogen bonding that...
8 KB (969 words) - 21:58, 27 February 2022
stereochemistry of the double bond. The presence of alkoxyl or related chelating groups accelerates lithium–halogen exchange. Lithium halogen exchange is typically...
10 KB (1,200 words) - 17:34, 25 September 2023
Bent's rule (category Chemical bonding)
2019-08-15. Retrieved 2023-12-06. Grabowski, Sławomir J. (2011-11-10). "Halogen Bond and Its Counterparts: Bent's Rule Explains the Formation of Nonbonding...
38 KB (4,250 words) - 13:50, 4 October 2024
Bromine (category Halogens)
afforded by the radial-nodeless 3d orbitals. Like the other carbon–halogen bonds, the C–Br bond is a common functional group that forms part of core organic...
67 KB (7,719 words) - 23:39, 21 November 2024