In organic chemistry, hyperconjugation (σ-conjugation or no-bond resonance) refers to the delocalization of electrons with the participation of bonds of...
20 KB (2,394 words) - 12:04, 1 May 2024
In organic chemistry, negative hyperconjugation is the donation of electron density from a filled π- or p-orbital to a neighboring σ*-orbital. This phenomenon...
2 KB (230 words) - 13:04, 27 February 2024
Anomeric effect (section Hyperconjugation)
widely accepted explanation is that there is a stabilizing interaction (hyperconjugation) between the unshared electron pair on the endocyclic heteroatom (within...
19 KB (2,539 words) - 18:53, 1 August 2024
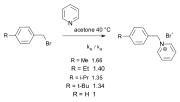
Negative hyperconjugation is a theorized phenomenon in organosilicon compounds, in which hyperconjugation stabilizes or destabilizes certain accumulations...
11 KB (1,170 words) - 21:01, 11 March 2024
substituted the carbocation, the more stable it is, due to induction and hyperconjugation. The major product of the addition reaction will be the one formed...
9 KB (1,129 words) - 04:58, 22 February 2024
are two main explanations for the gauche effect: hyperconjugation and bent bonds. In the hyperconjugation model, the donation of electron density from the...
15 KB (1,701 words) - 19:03, 21 September 2024
is perhaps the strongest candidate, with the stabilizing effect of hyperconjugation on the staggered conformation contributing to the phenomenon. Theoretical...
29 KB (2,858 words) - 11:46, 29 October 2024
interactions of the substituents as well as orbital interactions such as hyperconjugation are responsible for the relative stability of conformers and their...
33 KB (3,846 words) - 18:11, 24 July 2024
are two main explanations for the gauche effect: hyperconjugation and bent bonds. In the hyperconjugation model, the donation of electron density from the...
7 KB (865 words) - 08:42, 22 May 2024
syn-periplanar. The parallel orbitals can overlap and become involved in hyperconjugation. If the bonding orbital is an electron donor and the anti-bonding orbital...
11 KB (980 words) - 07:49, 9 May 2024
p orbital. Hyperconjugation is commonly invoked to explain the stability of alkyl substituted radicals and carbocations. Hyperconjugation is less important...
35 KB (4,303 words) - 03:15, 7 October 2024
weaker form of delocalization is hyperconjugation. In radical chemistry, radicals are stabilized by hyperconjugation with adjacent alkyl groups. The donation...
40 KB (4,613 words) - 06:59, 17 October 2024
rather than the S-trans (or E) alternative, due to a combination of hyperconjugation and dipole minimization effects. The preference for the Z conformation...
46 KB (4,840 words) - 14:46, 25 October 2024
of mixing full and empty orbitals to delocalize electrons, known as hyperconjugation. When the highest occupied molecular orbital (HOMO) of one system and...
14 KB (1,895 words) - 22:59, 25 June 2023
separation between alkyl groups is greatest in the most substituted alkene. Hyperconjugation, which describes the stabilizing interaction between the HOMO of the...
14 KB (1,543 words) - 11:38, 6 November 2023
that the reaction proceeds through a vinyl cation transition state. Hyperconjugation and hydrogen bonding was evoked to explain the accessibility of the...
38 KB (4,374 words) - 15:25, 1 September 2024
in other words, electron delocalization. Hyperconjugation Carbon radicals are stabilized by hyperconjugation, meaning that more substituted carbons are...
7 KB (863 words) - 23:59, 4 October 2024
also occurs in carbon compounds, where it is sometimes referred to as hyperconjugation; another name for asymmetrical three-center two-electron bonds. The...
5 KB (688 words) - 12:00, 26 March 2023
carbocations, and primary carbocations are the least stable(due to hyperconjugation). An equimolar mixture of ZnCl2 and concentrated HCl is the reagent...
4 KB (497 words) - 19:37, 3 September 2024
superacid media. The stabilization by alkyl groups is explained by hyperconjugation. The donation of electron density from a β C-H or C-C bond into the...
25 KB (2,969 words) - 13:41, 25 October 2024
show that 1,2-difluoroethane prefers the gauche conformation due to hyperconjugation effects. Since F is much more electronegative than the C atom, it will...
12 KB (1,105 words) - 03:54, 12 January 2024
promote fragmentation by stabilizing carbocation formation through hyperconjugation. As shown in the above picture, the "stable" carbocation is formed...
14 KB (1,418 words) - 18:35, 26 September 2024
overall activation energy. The lowering of the LUMO is the result of hyperconjugation between alkyne π donor orbitals and CF σ* acceptors. These interactions...
44 KB (5,098 words) - 05:29, 19 February 2024
determine the stability of reactants and products such as conjugation, hyperconjugation and aromaticity and the presence and stability of reactive intermediates...
14 KB (1,182 words) - 20:28, 22 July 2024
terms of a conjugation effect and in later years after the advent of hyperconjugation (1939) as its predecessor. A fundamental problem with the effect is...
4 KB (504 words) - 12:17, 9 April 2024
well-studied example of delocalization that does not involve π electrons (hyperconjugation) can be observed in the non-classical 2-Norbornyl cation Another example...
42 KB (5,094 words) - 06:04, 25 June 2024
of the non-bonding orbital at phosphorus through n(P) → σ*(Si-Si) hyperconjugation is more effective after metalation. This is due to the higher negative...
31 KB (3,350 words) - 18:13, 20 November 2023
carbocation is also stabilized by both inductive stabilization and hyperconjugation from attached alkyl groups. The Hammond–Leffler postulate suggests...
15 KB (1,970 words) - 03:54, 24 October 2024
softness-hardness-based models, aromaticity and antiaromaticity, hyperconjugation, etc. The application to main-group element compounds utilizes principles...
11 KB (1,244 words) - 14:54, 9 June 2024
hydrogen bonding, inductive effects, isomerism, resonance, aromaticity, hyperconjugation, mesomerism, carbocations and carbanions, free radical, bond cleavage...
55 KB (4,049 words) - 14:27, 7 November 2024