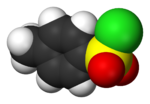
toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula −SO2−C6H4−CH3. It consists of a tolyl group, −C6H4−CH3...
8 KB (876 words) - 04:29, 2 December 2024
P-Toluenesulfonic acid (category P-Tosyl compounds)
alcohols, and other polar organic solvents. The CH3C6H4SO2 group is known as the tosyl group and is often abbreviated as Ts or Tos. Most often, TsOH refers...
9 KB (640 words) - 16:59, 22 October 2024
final step the reaction medium is neutralized to pH 7 (magenta) the tosyl group is eliminated as well. Ishikawa, H.; Suzuki, T.; Hayashi, Y. (2009)....
3 KB (322 words) - 16:57, 18 July 2023
Tosyl azide is a reagent used in organic synthesis. Tosyl azide is used for the introduction of azide and diazo functional groups. It is also used as...
2 KB (171 words) - 14:21, 28 February 2024
anion of p-bromophenylsulfonic acid (BrC6H4SO−3). Tosyl group Tosylic acid Triflic acid Sulfonyl group Smith, Michael B.; March, Jerry (2007). March's Advanced...
1 KB (91 words) - 22:49, 7 October 2024
Tosyl phenylalanyl chloromethyl ketone (TPCK) is a protease inhibitor. Its structural formula is 1-chloro-3-tosylamido-4-phenyl-2-butanone. TPCK is an...
3 KB (239 words) - 10:51, 4 September 2024
4-Toluenesulfonyl chloride (redirect from Tosyl chloride)
functional group. In characteristic manner, TsCl converts alcohols (abbreviated ROH) into the corresponding toluenesulfonate esters, or tosyl derivatives...
6 KB (422 words) - 16:14, 28 July 2024
cyclization then forms the 5-membered ring, which reacts to eliminate the tosyl group. The last step is tautomerization to the pyrrole.[citation needed] By...
33 KB (3,142 words) - 17:38, 21 February 2024
the substrate is an aldehyde, then elimination of the excellent tosyl leaving group can occur readily. Upon quenching, the resulting molecule is an oxazole...
4 KB (267 words) - 22:21, 27 September 2024
coupled with simple alkenes to form five, six or eight membered rings. Tosyl groups can be removed from N-tosylamides almost instantaneously, using SmI2...
11 KB (958 words) - 20:35, 17 September 2024
Tosylhydrazone (category P-Tosyl compounds)
is a functional group with the general structure RR'C=N-NH-Ts where Ts is a tosyl group. Organic compounds having this functional group can be accessed...
5 KB (657 words) - 15:37, 7 August 2024
Troc (trichloroethyl chloroformate ) group – Removed by Zn insertion in the presence of acetic acid Tosyl (Ts) group – Removed by concentrated acid (HBr...
57 KB (6,755 words) - 02:59, 9 November 2024
Concerns that the proposed symbol Ts may clash with a notation for the tosyl group used in organic chemistry were rejected, following existing symbols bearing...
70 KB (11,042 words) - 16:06, 4 January 2025
typical catalyst is (cymene)R,R-HNCHPhCHPhNTs, where Ts refers to a tosyl group (SO2C6H4Me) and R,R refers to the absolute configuration of the two chiral...
13 KB (1,292 words) - 14:45, 29 February 2024
converting the alcohol groups to tosylate groups with tosyl chloride and pyridine. The tosyl group then served as leaving groups in another reaction with...
58 KB (6,913 words) - 18:27, 31 December 2024
3-thiadiazoles through the reaction of hydrazone derivatives with an N-acyl or N-tosyl group reacted with thionyl chloride. An analogous reaction gives 1,2,3-selenadiazoles...
2 KB (200 words) - 22:09, 22 September 2024
Ketenyl anion (category Functional groups)
R group is more electron-withdrawing group, it becomes more likely to leave than tosyl group. For example, changing R group from cyclohexyl group (Cy)...
20 KB (2,090 words) - 21:19, 19 March 2024
electrophilic N-tosylimine. The sulfoxide group is removed by hydrogenation with Raney nickel. ts is a tosyl group, ee stands for enantiomeric excess Contra-Friedel–Crafts...
9 KB (1,041 words) - 06:11, 2 November 2024
with tosyl groups. The product is treated with 1,4-bis(brommethyl)benzene and potassium carbonate in acetonitrile. After cleaving of the tosyl groups with...
17 KB (1,685 words) - 07:47, 1 July 2024
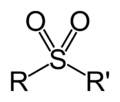
group is either a functional group found primarily in sulfones, or a substituent obtained from a sulfonic acid by the removal of the hydroxyl group,...
2 KB (197 words) - 10:47, 9 October 2024
hydrogen iodide and red phosphorus removed the tosyl group and hydrolysed both remaining ester groups to form diacid 13. Acetylation and esterification...
39 KB (4,087 words) - 03:50, 29 February 2024
Hydrazone (redirect from Tosyl hydrazone)
aldehydes by the replacement of the oxygen =O with the =N−NH2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes...
11 KB (1,154 words) - 13:23, 20 October 2024
completely missing an X chromosome Tennessine, symbol Ts, a chemical element Tosyl, a group in organic chemistry Transition state, of a chemical reaction Adobe...
3 KB (413 words) - 12:18, 31 December 2024
original series of a particular media, in contrast to a spin-off Tosyl, a chemical group Gy's sampling theory (abbreviation) Star Trek: The Original Series...
2 KB (286 words) - 18:49, 6 December 2024
Aziridines (category IARC Group 2B carcinogens)
cycloaddition. When the N-substituent is an electron-withdrawing group such as a tosyl group, the carbon-nitrogen bond breaks, forming another zwitterion...
16 KB (1,680 words) - 13:29, 4 September 2024
Skeletal formula (section Alkyl groups)
the use of Ts to represent tosyl never causes confusion); OTs is the tosylate group A protecting group or protective group is introduced into a molecule...
28 KB (3,597 words) - 19:40, 16 December 2024
1016/S0040-4039(01)81842-6. Hegedus, L. S.; Holden, M. S.; McKearin, J. M. (1984). "cis-N-TOSYL-3-METHYL-2-AZABICYCLO[3.3.0]OCT-3-ENE". Organic Syntheses. 62: 48; Collected...
15 KB (1,591 words) - 22:35, 30 October 2024
EDTA or by other serine protease inhibitors like Nα-Tosyl-Lys Chloromethyl Ketone (TLCK) and Nα-Tosyl-Phe Chloromethyl Ketone (TPCK)[citation needed]. Protease...
10 KB (1,063 words) - 20:45, 11 September 2024
Sulfonyl halide (category Functional groups)
C6H6 → RSO2C6H5 + HCl A readily available arylsulfonyl chloride source is tosyl chloride. The desulfonation of arylsulfonyl chlorides provides a route to...
14 KB (1,485 words) - 20:44, 17 December 2024
Alcohol (chemistry) (redirect from Tertiary hydroxyl groups)
H2O}}} Other types of ester are prepared in a similar manner−for example, tosyl (tosylate) esters are made by reaction of the alcohol with 4-toluenesulfonyl...
35 KB (3,855 words) - 08:18, 21 November 2024