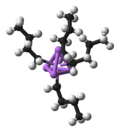
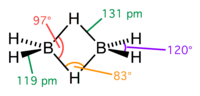
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some...
23 KB (2,641 words) - 01:22, 5 October 2024
bridging hydrides. Classical transition metal hydride feature a single bond between the hydrogen centre and the transition metal. Some transition metal hydrides...
21 KB (2,300 words) - 05:20, 19 November 2024
Palladium hydride is palladium metal with hydrogen within its crystal lattice. Despite its name, it is not an ionic hydride but rather an alloy of palladium...
21 KB (2,800 words) - 13:16, 14 November 2024
Metal carbonyl hydrides are complexes of transition metals with carbon monoxide and hydride as ligands. These complexes are useful in organic synthesis...
6 KB (624 words) - 07:31, 25 June 2024
Sodium hydride is the chemical compound with the empirical formula NaH. This alkali metal hydride is primarily used as a strong yet combustible base in...
14 KB (1,339 words) - 02:55, 29 March 2024
Potassium hydride, KH, is the inorganic compound of potassium and hydrogen. It is an alkali metal hydride. It is a white solid, although commercial samples...
5 KB (422 words) - 18:59, 18 January 2024
Iron tetracarbonyl dihydride (redirect from Iron carbonyl hydride)
compound with the formula H2Fe(CO)4. This compound was the first transition metal hydride discovered. The complex is stable at low temperatures but decomposes...
8 KB (700 words) - 07:26, 25 June 2024
has made significant contributions to, projects dealing with transition metal hydrides. He is currently Distinguished Professor at Indiana University...
6 KB (744 words) - 15:01, 7 June 2024
as post-transition metals, poor metals, other metals, p-block metals and chemically weak metals. The most common name, post-transition metals, is generally...
124 KB (15,306 words) - 16:20, 28 October 2024
Lithium hydride is an inorganic compound with the formula LiH. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic...
19 KB (1,930 words) - 17:06, 27 October 2024
saline hydride, meaning that its structure is salt-like. The alkali metals and the alkaline earth metals heavier than beryllium all form saline hydrides. A...
7 KB (681 words) - 01:24, 5 November 2024
Organometallic chemistry (redirect from Metal carbon bonding)
carbonyl hydride is used in the commercial production of many aldehyde-based fragrances. Zeise's salt is an example of a transition metal alkene complex...
31 KB (3,157 words) - 20:03, 24 September 2024
temperature; despite this it is the most stable of the binary first row transition metal hydrides. A variety of coordination compounds containing Zn–H bonds are...
8 KB (912 words) - 12:36, 9 April 2024
N-allylamides. LAH is widely used to prepare main group and transition metal hydrides from the corresponding metal halides. LAH also reacts with many inorganic ligands...
35 KB (3,052 words) - 14:36, 12 July 2024
Aluminium hydride (also known as alane and alumane) is an inorganic compound with the formula AlH3. Alane and its derivatives are part of a family of...
32 KB (3,312 words) - 05:12, 9 October 2024
Titanium hydride normally refers to the inorganic compound TiH2 and related nonstoichiometric materials. It is commercially available as a stable grey/black...
15 KB (1,764 words) - 11:41, 28 June 2024
Diborane (redirect from Boron hydride)
(1978). "Ch. 17. Vibrational spectroscopy of hydride-bridged transition metal compounds". Transition Metal Hydrides. Advances in Chemistry. Vol. 167. pp. 232–247...
27 KB (2,600 words) - 19:52, 29 October 2024
organic matter and is a greenhouse gas. The other hydrides are generally unstable, poisonous metal hydrides. They take on a pyramidal structure, and as such...
9 KB (818 words) - 10:32, 15 November 2024
1016/S0360-3199(00)00037-9. Lummis, Paul Ashley (2015). Earth-Abundant Transition Metal Hydride Complexes for Dehydrocoupling and Hydrosilylation Catalysis. University...
10 KB (757 words) - 09:15, 1 January 2024
Pnictogen hydrides or hydrogen pnictides are binary compounds of hydrogen with pnictogen (/ˈpnɪktədʒən/ or /ˈnɪktədʒən/; from Ancient Greek: πνῑ́γω "to...
8 KB (664 words) - 01:17, 15 November 2024
Boranes (section Binary boron hydrides)
the anionic boron hydrides and the related aluminium hydrides. Schlesinger's work laid the foundation for a host of boron hydride reagents for organic...
5 KB (561 words) - 16:24, 21 October 2024
square planar. Stryker's reagent, [(PPh3)CuH]6, PPh3-stabilized transition metal hydride cluster that used as a reagent for "conjugate reductions"....
14 KB (1,418 words) - 17:55, 22 June 2024
1021/ja981965+. King, R.B. (2000). "Structure and bonding in homoleptic transition metal hydride anions". Coordination Chemistry Reviews. 200–202: 813–829. doi:10...
22 KB (2,870 words) - 20:18, 28 October 2024
presence of a formal hydride ion, the oxidation state of cobalt in this compound is +1. But unlike some other transition-metal hydrides complexes, HCo(CO)4...
9 KB (806 words) - 07:19, 25 June 2024
palladium hydride) may be due to the formation and migration of polonium hydride. Polonium hydride is a more covalent compound than most metal hydrides because...
6 KB (534 words) - 16:07, 27 January 2024
The helium hydride ion, hydridohelium(1+) ion, or helonium is a cation (positively charged ion) with chemical formula HeH+. It consists of a helium atom...
30 KB (2,989 words) - 04:40, 1 November 2024
Hydrogen halide (redirect from Group 17 hydride)
CH3CH2Cl Pseudohalogen Hypohalous acid group 13 hydrides group 14 hydrides group 15 hydrides group 16 hydrides Greenwood, Norman N.; Earnshaw, Alan (1997)...
7 KB (616 words) - 18:49, 21 March 2024
β-Hydride elimination is a reaction in which an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride and an alkene...
4 KB (572 words) - 18:43, 21 November 2024
Boron hydride clusters are compounds with the formula BxHy or related anions, where x ≥ 3. Many such cluster compounds are known. Common examples are...
13 KB (1,430 words) - 17:51, 20 August 2024
S. (2016). "Main Group Lewis Acid-Mediated Transformations of Transition-Metal Hydride Complexes". Chemical Reviews. 116 (15): 8873–8911. doi:10.1021/acs...
3 KB (374 words) - 12:16, 18 November 2022