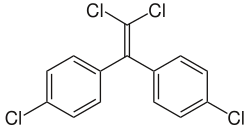
In chemistry, dehydrohalogenation is an elimination reaction which removes a hydrogen halide from a substrate. The reaction is usually associated with...
8 KB (858 words) - 02:11, 26 March 2025
Alkynes are prepared from 1,1- and 1,2-dihaloalkanes by double dehydrohalogenation. The reaction provides a means to generate alkynes from alkenes,...
24 KB (3,191 words) - 00:43, 2 March 2025
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular...
21 KB (2,204 words) - 17:08, 1 April 2025
refrigerant in air conditioners. It is prepared by fluorination and dehydrohalogenation reactions starting with 1,1,1,3,3-pentachloropropane. Cheryl Hogue...
3 KB (130 words) - 17:40, 7 January 2024
reacts in a coupling reaction with epichlorohydrin, followed by dehydrohalogenation. Epoxy resins produced from such epoxy monomers are called glycidyl-based...
67 KB (8,424 words) - 07:25, 14 April 2025
formation of urethane foams and epoxy resins. It is also useful in dehydrohalogenation reactions and Swern oxidations. Triethylamine is readily alkylated...
14 KB (1,090 words) - 01:39, 11 March 2025
Sodium amide (section Dehydrohalogenation)
which is recycled typically. Sodium amide is a standard base for dehydrohalogenations. It induces the loss of two equivalents of hydrogen bromide from...
14 KB (1,676 words) - 03:31, 11 March 2025
of trimethylaniline by dibromoethane followed by ring closure and dehydrohalogenation. Nolan, Steven P. (2006). N-Heterocyclic Carbenes in Synthesis. Wiley-VCH...
2 KB (127 words) - 05:37, 13 August 2024
Base-induced bimolecular dehydrohalogenation (an E2 type reaction mechanism). The optimum geometry for the transition state requires the breaking bonds...
30 KB (3,416 words) - 03:35, 17 March 2025
n-BuI, which can react with the RLi reagent formed, and by competing dehydrohalogenation reactions, in which n-BuLi serves as a base: 2 C4H9Br + RLi → 2 C4H9R...
18 KB (1,858 words) - 22:02, 6 April 2025
is a chemical compound formed by the loss of hydrogen chloride (dehydrohalogenation) from DDT, of which it is one of the more common breakdown products...
11 KB (951 words) - 00:49, 28 December 2024
well. In the presence of a strong base, alkyl chlorides undergo dehydrohalogenation to give alkenes or alkynes.[citation needed] Alkyl chlorides react...
18 KB (2,000 words) - 16:11, 13 March 2025
while iodine is the least reactive of them all. The facility of dehydrohalogenation follows the reverse trend: iodine is most easily removed from organic...
10 KB (1,113 words) - 22:59, 29 March 2025
terminal acetylenes and active methylene compounds. It is useful in dehydrohalogenation reactions. Illustrating the latter behavior, potassium tert-butoxide...
9 KB (703 words) - 09:57, 2 April 2025
triflate). When an alkyl halide is used, the reaction is called a dehydrohalogenation. For unsymmetrical products, the more substituted alkenes (those...
49 KB (5,264 words) - 02:55, 7 April 2025
chloramine with the formula C5H10NCl. The resulting chloramine undergoes dehydrohalogenation to afford the cyclic imine. 13C NMR: (CDCl3, ppm) 47, 27.2, 25.2[citation...
17 KB (1,474 words) - 02:38, 11 March 2025
[Specific proton acceptors as auxiliary bases in alkylation and dehydrohalogenation reactions]. Chemische Berichte (in German). 91 (2). Wiley-VCH: 380–392...
8 KB (582 words) - 03:25, 28 February 2025
with C70 and higher fullerenes, but not with C60. It is useful for dehydrohalogenations. 1,5-Diazabicyclo[4.3.0]non-5-ene DABCO Kaupmees, K.; Trummal, A...
7 KB (514 words) - 11:14, 8 March 2025
trichloroethane to give aluminium trichloride, which in turn catalyses the dehydrohalogenation of the remaining trichloroethane to vinylidene chloride and hydrogen...
24 KB (2,239 words) - 11:46, 13 March 2025
magnesium salts and an extended alkyl compound.[citation needed] In dehydrohalogenation reactions, the halogen and an adjacent proton are removed from halocarbons...
20 KB (2,475 words) - 08:11, 2 April 2025
as a source of the 2-propenyl group. One early synthesis involves dehydrohalogenation of 1,2-dichloropropane with potassium hydroxide. Takahashi, Tamotsu;...
2 KB (132 words) - 15:34, 3 March 2023
Decarbonylation Decarboxylation Dehydration Dehalogenation Dehydrogenation Dehydrohalogenation Deprotonation Desilylation Dimerisation Disproportionation Dötz reaction...
4 KB (263 words) - 17:12, 10 January 2025
used in situ rather than being isolated. The compound undergoes dehydrohalogenation to afford the cyclic imine. Claxton, George P.; Allen, Lloyd; Grisar...
2 KB (95 words) - 00:43, 21 July 2023
isobutyryl chloride is the subject of many reported transformations. Dehydrohalogenation of isobutyryl chloride with triethylamine gives 2,2,4,4-tetramethylcyclobutanedione...
2 KB (140 words) - 14:44, 8 February 2025
empirical rule for predicting the favored regiochemistry in the dehydrohalogenation of alkyl iodides, though it turns out that the rule is applicable...
14 KB (1,543 words) - 11:38, 6 November 2023
propargylic halides by SN2′ displacement by an organocuprate from dehydrohalogenation of certain dihalides from reaction of a triphenylphosphinyl ester...
38 KB (4,126 words) - 18:33, 1 April 2025
brominated, and the resulting dibromodiphenylethane is subjected to dehydrohalogenation. Yet another method involves the coupling of iodobenzene and the...
5 KB (304 words) - 21:13, 31 December 2023
diastereoisomer predominates). An example of modest stereoselectivity is the dehydrohalogenation of 2-iodobutane which yields 60% trans-2-butene and 20% cis-2-butene...
8 KB (1,005 words) - 19:52, 2 April 2025
otherwise it dimerises to biphenylene. Early routes to benzyne involved dehydrohalogenation of aryl halides: Such reactions require strong base and high temperatures...
28 KB (3,128 words) - 22:43, 22 March 2025
cycloaddition with dichloro ketene diazomethane insertion reaction dehydrohalogenation reaction with DMF Luche reduction to alcohol with sodium borohydride...
13 KB (1,221 words) - 14:23, 24 March 2025