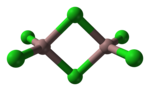
Gallium(III) bromide (GaBr3) is a chemical compound, and one of four gallium trihalides. Gallium(III) bromide is, at room temperature and atmospheric...
9 KB (869 words) - 02:13, 20 October 2024
Gallium(III) chloride is an inorganic chemical compound with the formula GaCl3 which forms a monohydrate, GaCl3·H2O. Solid gallium(III) chloride is a deliquescent...
15 KB (1,386 words) - 13:22, 12 July 2024
Gold(I) bromide can be formed by synthesis from the elements or partial decomposition of gold(III) bromide by careful control of temperatures and pressures...
4 KB (359 words) - 13:11, 14 April 2023
number GaAs gallium(III) arsenide 1303-00-0 GaAsO4 gallium(III) orthoarsenate GaBr3 gallium(III) bromide 13450-88-9 Ga(C2H3O2)3 gallium(III) acetate 2571-06-4...
183 KB (107 words) - 22:11, 6 September 2024
Their proper names are gallium(III) fluoride, gallium(III) chloride, gallium(III) bromide and gallium(III) iodide. GaF3 GaF3 is a white solid which sublimes...
7 KB (839 words) - 09:15, 2 May 2024
Thallium halides (redirect from Thallium(III) bromide)
prepared in CH3CN by treating a solution of TlCl with Cl2 gas. Thallium(III) bromide This unstable compound decomposes at less than 40 °C to TlBr and Br2...
14 KB (1,634 words) - 10:17, 8 June 2024
Aluminium bromide is any chemical compound with the empirical formula AlBrx. Aluminium tribromide is the most common form of aluminium bromide. It is a...
10 KB (631 words) - 11:21, 14 July 2024
– GaP Gallium(II) sulfide – GaS Gallium(III) sulfide – Ga2S3 Digermane – Ge2H6 Germane – GeH4 Germanium(II) bromide – GeBr2 Germanium(II) chloride –...
119 KB (8,735 words) - 17:31, 25 October 2024
CAS number GaAs gallium(III) arsenide 1303–00–0 GaBr3 gallium(III) bromide 13450–88–9 GaCl2 gallium(II) chloride 24597–12–4 GaCl3 gallium trichloride 13450–90–3...
139 KB (120 words) - 17:07, 15 July 2024
List of semiconductor materials (redirect from III-V semiconductor)
can form binary (two elements, e.g. gallium(III) arsenide (GaAs)), ternary (three elements, e.g. indium gallium arsenide (InGaAs)) and quaternary alloys...
54 KB (2,525 words) - 05:44, 8 October 2024
ISBN 978-0-08-037941-8. Anthony John Downs (1993). Chemistry of aluminium, gallium, indium, and thallium. Springer. ISBN 0-7514-0103-X. Main Group Metals...
8 KB (671 words) - 22:12, 9 August 2024
Bromine (section Hydrogen bromide)
While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br−) has caused its accumulation in the oceans. Commercially the element...
67 KB (7,719 words) - 01:26, 30 October 2024
Ammonium hexafluoroindate (redirect from Ammonium hexafluoroindate(III))
The compound can be obtained by reacting ammonium fluoride and indium bromide in anhydrous methanol, or by reacting ammonium fluoride and indium fluoride...
4 KB (269 words) - 19:07, 12 September 2024
of the more effective methods. Plutonium(III) fluoride can be used for manufacture of the plutonium-gallium alloy instead of more difficult to handle...
5 KB (300 words) - 13:00, 21 December 2023
Indium halides (section Indium(III) bromide)
+3, and their proper names are indium(III) fluoride, indium(III) chloride, indium(III) bromide, and indium(III) iodide. The trihalides are Lewis acidic...
20 KB (2,262 words) - 09:09, 2 May 2024
Aluminium iodide (redirect from Aluminium(III) iodide)
with hydrogen iodide or hydroiodic acid. Like the related chloride and bromide, AlI 3 is a strong Lewis acid and will absorb water from the atmosphere...
7 KB (484 words) - 14:40, 19 January 2024
doi:10.1016/0022-1902(71)80004-0. S. J. Patel; D. G. Tuck (1969). "Gallium(III) isothiocyanate and its addition compounds". Canadian Journal of Chemistry...
35 KB (1,083 words) - 11:43, 19 May 2024
Indium (section Indium(III))
metal and one of the softest elements. Chemically, indium is similar to gallium and thallium, and its properties are largely intermediate between the two...
49 KB (5,839 words) - 19:45, 30 October 2024
between the monomers. Trialkyl thallium compounds, like those of indium and gallium, can be prepared from thallium trihalides and Grignard reagents or organolithium...
9 KB (930 words) - 00:20, 13 August 2024
Thallium (section Thallium(III))
state resembles that of the other elements in group 13 (boron, aluminium, gallium, indium). However, the +1 state, which is far more prominent in thallium...
52 KB (5,787 words) - 13:10, 4 November 2024
Aluminium chloride (redirect from Aluminium(III) chloride)
hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour. The anhydrous form is commercially...
23 KB (1,721 words) - 09:46, 18 July 2024
chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group 17. The...
52 KB (5,499 words) - 18:00, 11 September 2024
zincblende itself, lead(II) nitrate, many compound semiconductors (such as gallium arsenide and cadmium telluride), and a wide array of other binary compounds...
49 KB (3,620 words) - 14:56, 4 October 2024
Gallium monoiodide is an inorganic gallium compound with the formula GaI or Ga4I4. It is a pale green solid and mixed valent gallium compound, which can...
34 KB (3,439 words) - 03:33, 22 August 2024
germanium, the Ga–As bond in gallium arsenide, the Zn–Se bond in zinc selenide and the Cu–Br bond in copper(I) bromide. Grimm, H.G.; Sommerfeld, A (1926)...
2 KB (277 words) - 21:46, 15 July 2022
(space group R3). The bromide is an exception with the orthorhombic PuBr3-type structure and space group Cmcm. Crystals of americium(III) chloride hexahydrate...
77 KB (9,329 words) - 07:44, 1 November 2024
Copper (redirect from Copper(III))
Metallic Compounds on the Reaction between Isophorone and Methylmagnesium Bromide". Journal of the American Chemical Society. 63 (9): 2308–2316. doi:10.1021/ja01854a005...
122 KB (13,910 words) - 18:45, 24 October 2024
Diborane (redirect from Boron(III) hydride)
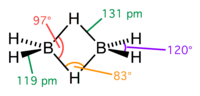
compounds with such unusual bonding. Of the other elements in group IIIA, gallium is known to form a similar compound digallane, Ga2H6. Aluminium forms a...
27 KB (2,600 words) - 19:52, 29 October 2024
mineral acids are rapid. Californium is only water-soluble as the californium(III) cation. Attempts to reduce or oxidize the +3 ion in solution have failed...
44 KB (4,631 words) - 23:46, 21 August 2024
Boron trichloride (redirect from Boron(III) chloride)
reagent in the synthesis of organic compounds. Like the corresponding bromide, it cleaves C-O bonds in ethers. BCl3 is an aggressive reagent that can...
10 KB (951 words) - 09:31, 27 April 2024