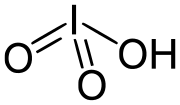
Iodic acid is a white water-soluble solid with the chemical formula HIO3. Its robustness contrasts with the instability of chloric acid and bromic acid...
8 KB (681 words) - 00:26, 22 May 2024
Sodium iodate (redirect from Iodic acid sodium salt)
Sodium iodate (NaIO3) is the sodium salt of iodic acid. Sodium iodate is an oxidizing agent. It has several uses. It can be prepared by reacting a sodium-containing...
5 KB (228 words) - 14:09, 24 January 2024
Potassium iodate (redirect from Iodic acid potassium salt)
reacting a potassium-containing base such as potassium hydroxide with iodic acid, for example: HIO3 + KOH → KIO3 + H2O It can also be prepared by adding...
11 KB (746 words) - 10:30, 18 April 2024
two forms: orthoperiodic acid, with the chemical formula H5IO6, and metaperiodic acid, which has the formula HIO4. Periodic acid was discovered by Heinrich...
12 KB (912 words) - 14:02, 29 April 2024
Iodine pentoxide (redirect from Iodic anhydride)
iodine oxide is the anhydride of iodic acid, and the only stable oxide of iodine. It is produced by dehydrating iodic acid at 200 °C in a stream of dry air:...
4 KB (261 words) - 05:53, 17 March 2023
compound can be prepared by reacting sulphuric acid with iodic acid or by the action of concentrated nitric acid upon dry powedered iodine. It forms yellow...
4 KB (268 words) - 08:45, 11 September 2023
"period", but from "iodine": per-iodic acid (compare iodic acid, perchloric acid), and it is thus pronounced per-iodic /ˌpɜːraɪˈɒdɪk/ PUR-eye-OD-ik, and...
41 KB (4,896 words) - 02:07, 5 July 2024
purpose of adding the nitric acid is to oxidise the carbon and hydrogen. Concentrated nitric acid only oxidises iodine to iodic acid and doesn't affect any...
1 KB (190 words) - 13:44, 9 January 2023
hypochlorous acid, producing chloric acid and hydrogen chloride: 3 HClO → HClO3 + 2 HCl Chlorate Hypochlorous acid Chlorous acid Perchloric acid Oxidizing acid Dichlorine...
4 KB (243 words) - 15:24, 3 November 2023
radium and iodic acid with the chemical formula Ra(IO3)2. Radium iodate is obtained by the reaction of a soluble radium salt and iodic acid:[citation needed]...
3 KB (174 words) - 21:22, 18 January 2024
important are the four oxoacids: hypoiodous acid (HIO), iodous acid (HIO2), iodic acid (HIO3), and periodic acid (HIO4 or H5IO6). When iodine dissolves in...
106 KB (11,803 words) - 10:18, 11 July 2024
schwefliger Säure" [On the duration of the reaction between iodic acid and sulfurous acid]. Berichte der Deutschen Chemischen Gesellschaft (in German)...
9 KB (991 words) - 14:03, 26 June 2024
obtained in 2020 through the hydrothermal reaction of tin(II) oxide and iodic acid in water at 220 °C. It is a colorless columnar crystal, crystallized in...
2 KB (170 words) - 19:50, 15 July 2024
obtained by the reaction of dysprosium nitrate or dysprosium chloride and iodic acid at 200 °C. It exists in two crystal forms: α-form and β-form. Its solubility...
2 KB (173 words) - 15:52, 2 May 2024
obtained by reacting bismuth nitrate and iodic acid, dissolving the resulting precipitate in 7.8 mol/L nitric acid, and heating to volatilize and crystallize...
3 KB (199 words) - 22:38, 12 May 2024
hydrothermal reaction of europium(III) nitrate or europium(III) oxide and iodic acid in water at 230 °C. It can be thermally decomposed as follows: 7 Eu(IO3)3...
2 KB (160 words) - 01:25, 29 March 2024
Oxyacid (redirect from Oxygen acid)
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one...
22 KB (1,761 words) - 14:37, 5 July 2024
the salts can be prepared from preformed hypervalent iodines such as iodic acid, iodosyl sulfate or iodosyl triflate. The first such compound was synthesised...
18 KB (1,940 words) - 17:18, 30 June 2024
important are the four oxoacids: hypoiodous acid (HIO), iodous acid (HIO2), iodic acid (HIO3), and periodic acid (HIO4 or H5IO6). When iodine dissolves in...
30 KB (3,546 words) - 15:26, 10 July 2024
chromate iodates. Iodate sulfates can be produced from water solutions of iodic acid and sulfate salts. Lu, Huangjie; Guo, Xiaojing; Wang, Yaxing; Diefenbach...
12 KB (783 words) - 13:23, 12 January 2024
540 °C. It can be generated in the reaction of plutonium(IV) nitrate and iodic acid, but this method cannot obtain a pure product; Another preparation method...
3 KB (214 words) - 22:34, 12 May 2024
I2 + 9 O3 → I4O9 + 9 O2 It can also be produced by heating iodic acid and phosphoric acid together: 8 HIO3 → 2 I4O9 + 4 H2O + O2 Tetraiodine nonoxide...
2 KB (233 words) - 19:25, 28 February 2024
derivatives and hydrolysis of ethylene glycol with periodic acid. The produced iodic acid is detected with silver nitrate. Ethylene oxide is extremely...
108 KB (11,539 words) - 06:12, 9 July 2024
pentafluoride. 2IOF3 ⇌ IO2F + IF5 Dissolving the anhydride of iodic acid, I2O5, in anhydrous hydrofluoric acid. I2O5 + HF → IO2F + HIO3 Iodyl fluoride forms colorless...
4 KB (320 words) - 08:23, 26 May 2023
ozone depletion agents. Diiodine pentoxide (I2O5) is the anhydride of iodic acid and the only stable anhydride of an iodine oxoacid. Tetraiodine nonoxide...
8 KB (739 words) - 00:19, 3 January 2024
Iodine monochloride is soluble in acids such as HF and HCl but reacts with pure water to form HCl, iodine, and iodic acid: ICl + H2O → HCl + HI + 1⁄2O2 2...
6 KB (478 words) - 07:22, 4 December 2023
periodate and periodic acid in water at 160 °C, or by the hydrothermal reaction of terbium(III) nitrate or terbium(III) chloride and iodic acid at 200 °C . It...
3 KB (228 words) - 11:46, 20 March 2024
sulfonic acids sulphovinic acid, ethionic acid and isethionic acid and their salts; and, in cooperation with CF Ammermüller, of per-iodic acid and its...
10 KB (1,064 words) - 16:46, 10 May 2024
solution. He investigated the role of the iodate (IO− 3), the anion of iodic acid, in the catalytic conversion of hydrogen peroxide to oxygen and water...
5 KB (500 words) - 12:12, 2 May 2022