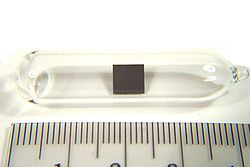
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with...
225 KB (24,164 words) - 20:18, 19 June 2025
scientific applications, in domestic water softeners (as a substitute for sodium chloride salt), as a feedstock, and in food processing, where it may be...
32 KB (3,033 words) - 20:49, 26 May 2025
This list contains fictional chemical elements, materials, isotopes or subatomic particles that either a) play a major role in a notable work of fiction...
96 KB (2,258 words) - 21:16, 6 June 2025
Iodine-131 (category Isotopes of iodine)
half-life longer than hours, since most lighter isotopes of tellurium become heavier stable isotopes, or else stable iodine or xenon. However, the heaviest...
39 KB (4,377 words) - 01:55, 20 May 2025
magma about 525 million years ago during the Cambrian period. A study of sodium-rich quartz-alkali feldspar—biotite gneiss granulite facies terrane in the...
4 KB (403 words) - 13:03, 27 June 2024
Plutonium (section Isotopes and nucleosynthesis)
to power some spacecraft. Plutonium isotopes are expensive and inconvenient to separate, so particular isotopes are usually manufactured in specialized...
140 KB (15,294 words) - 13:25, 24 June 2025
Praseodymium (section Isotopes)
isotopes lighter than 141Pr is positron emission or electron capture to isotopes of cerium, while that of heavier isotopes is beta decay to isotopes of...
38 KB (5,173 words) - 23:46, 19 June 2025
substances and investigations into isotopes". 1922: Aston receives the Nobel Prize in Chemistry "for his discovery of isotopes in a large number of non-radioactive...
30 KB (3,721 words) - 11:57, 5 June 2025
Soapstone Soda niter Sodium Sodium bicarbonate Sodium carbonate Sodium chloride Sodium citrate Sodium cyanide Sodium hydroxide Sodium hypochlorite Solid...
23 KB (1,957 words) - 02:53, 22 January 2025
detonations above, or in, the ocean, will contain short-lived radioactive sodium isotopes, and salts from the sea water. Molten silica is a very good solvent...
43 KB (5,527 words) - 05:46, 19 June 2025
that an unknown country had dispersed large quantities of radioactive sodium isotopes into the atmosphere. The resulting lethal dust cloud spread around...
16 KB (1,996 words) - 20:16, 21 June 2025
later wrote, "seemed to be whether Fermi had found isotopes of transuranian elements, or isotopes of the next-lower element, protactinium. At that time...
100 KB (12,681 words) - 08:16, 24 June 2025