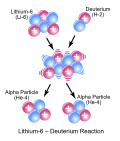The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based...
33 KB (4,076 words) - 22:52, 11 September 2024
The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei composed of...
15 KB (1,937 words) - 00:58, 28 August 2024
Atom (redirect from Atomic chemical)
are attracted to the protons in an atomic nucleus by the electromagnetic force. The protons and neutrons in the nucleus are attracted to each other by the...
125 KB (12,762 words) - 12:16, 16 September 2024
Mass number (redirect from Atomic mass number)
nucleons) in an atomic nucleus. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. Since...
8 KB (1,101 words) - 02:39, 15 April 2024
Nuclear shell model (redirect from Shell model of the atomic nucleus)
rules for the ordering of the nucleus shells are similar to Hund's Rules of the atomic shells, however, unlike its use in atomic physics, the completion of...
30 KB (4,119 words) - 16:59, 28 August 2024
The shape of the atomic nucleus depends on the variety of factors related to the size and shape of its nucleon constituents and the nuclear force holding...
29 KB (3,337 words) - 06:33, 31 August 2024
hydrogen atom, and the atomic number had been identified as the charge on the nucleus.: §1.1.2 Throughout the 1920s, the nucleus was viewed as composed...
76 KB (8,780 words) - 18:02, 15 September 2024
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. Atomic physics typically refers to...
9 KB (1,123 words) - 20:41, 9 September 2024
up nucleus in Wiktionary, the free dictionary. Nucleus (pl.: nuclei) is a Latin word for the seed inside a fruit. It most often refers to: Atomic nucleus...
3 KB (368 words) - 06:33, 20 June 2024
Rutherford model (redirect from Rutherford Atomic Model)
containing most of the atom's mass; this region would be known as the atomic nucleus. The Rutherford model was subsequently superseded by the Bohr model...
15 KB (1,719 words) - 23:34, 12 September 2024
Neutron (section Neutrons in an atomic nucleus)
behave similarly within the nucleus, they are both referred to as nucleons. Nucleons have a mass of approximately one atomic mass unit, or dalton (symbol:...
115 KB (12,955 words) - 22:03, 26 September 2024
The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the...
28 KB (1,808 words) - 08:11, 25 September 2024
Semi-empirical mass formula (redirect from Liquid drop model of the atomic nucleus)
the Bethe–Weizsäcker process) is used to approximate the mass of an atomic nucleus from its number of protons and neutrons. As the name suggests, it is...
26 KB (3,921 words) - 04:27, 26 July 2024
List of chemical elements (redirect from List of elements by atomic number)
of atom which has a specific number of protons in its atomic nucleus (i.e., a specific atomic number, or Z). The definitive visualisation of all 118...
2 KB (439 words) - 14:47, 25 September 2024
that the charge of an atomic nucleus, expressed as a multiplier of hydrogen's nuclear charge (qe), is roughly half the atom's atomic weight. In June 1911...
70 KB (9,130 words) - 22:30, 16 September 2024
Bohr model (redirect from Bohr's Atomic Theory)
Thomson only to be replaced by the quantum atomic model in the 1920s. It consists of a small, dense nucleus surrounded by orbiting electrons. It is analogous...
74 KB (10,099 words) - 01:45, 2 October 2024
Aufbau principle (redirect from Atomic buildup)
is used to predict the configuration of protons and neutrons in an atomic nucleus. In neutral atoms, the approximate order in which subshells are filled...
28 KB (3,074 words) - 23:52, 24 September 2024
possibility of atomic energy. H. G. Wells popularized the phrase "splitting the atom",[citation needed] before discovery of the atomic nucleus. Atomic energy...
1 KB (179 words) - 15:58, 12 September 2024
Nuclear reaction (redirect from Compound Nucleus)
the 6 3Li nucleus has a standard atomic weight of 6.015 atomic mass units (abbreviated u), the deuterium has 2.014 u, and the helium-4 nucleus has 4.0026...
20 KB (2,391 words) - 17:40, 25 June 2024
In nuclear physics, an atomic nucleus is called a halo nucleus or is said to have a nuclear halo when it has a core nucleus surrounded by a "halo" of orbiting...
9 KB (929 words) - 16:33, 2 September 2024
Magic number (physics) (redirect from Magic nucleus)
such that they are arranged into complete shells within the atomic nucleus. As a result, atomic nuclei with a "magic" number of protons or neutrons are much...
19 KB (2,235 words) - 18:34, 28 September 2024
decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or "decays" into a different atomic nucleus, with a mass number...
19 KB (2,542 words) - 00:16, 9 September 2024
high-speed electron or positron emitted by the radioactive decay of an atomic nucleus, known as beta decay. There are two forms of beta decay, β− decay and...
14 KB (1,522 words) - 15:16, 13 September 2024
Subatomic particle (redirect from Sub atomic particle)
an atomic nucleus, e.g. a helium-4 nucleus is composed of two protons and two neutrons. Most hadrons do not live long enough to bind into nucleus-like...
34 KB (3,156 words) - 13:13, 23 July 2024
Chemical bond (redirect from Atomic bond)
atom became clearer with Ernest Rutherford's 1911 discovery that of an atomic nucleus surrounded by electrons in which he quoted Nagaoka rejected Thomson's...
40 KB (4,872 words) - 13:33, 22 September 2024
physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming...
58 KB (6,985 words) - 03:44, 27 August 2024
"atmosphere" (the electron), distributed around a relatively tiny planet (the nucleus). Atomic orbitals exactly describe the shape of this "atmosphere" only when...
84 KB (10,942 words) - 19:19, 1 October 2024
the nucleus of their metals were (they had only just discovered the nucleus existed at all), but they assumed it was proportional to the atomic weight...
97 KB (13,069 words) - 16:58, 2 October 2024
dinitrogen in 1929 which provided the first experimental evidence that the atomic nucleus is not composed of protons and electrons, as was incorrectly believed...
9 KB (1,027 words) - 09:27, 30 June 2024
Nuclear structure (redirect from Structure of the atomic nucleus)
the structure of the atomic nucleus is one of the central challenges in nuclear physics. The cluster model describes the nucleus as a molecule-like collection...
33 KB (4,580 words) - 00:42, 14 December 2023