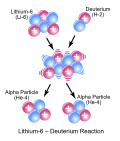The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration...
26 KB (3,716 words) - 19:46, 19 September 2024
kinetics, a reaction rate constant or reaction rate coefficient ( k {\displaystyle k} ) is a proportionality constant which quantifies the rate and direction...
17 KB (2,392 words) - 03:06, 31 May 2024
expression for the reaction rate of a given reaction in terms of concentrations of chemical species and constant parameters (normally rate coefficients and...
44 KB (7,596 words) - 01:53, 28 July 2024
Enzyme kinetics (redirect from Enzyme reaction rate)
Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactions. In enzyme kinetics, the reaction rate is measured and the effects of varying...
71 KB (9,341 words) - 03:06, 26 August 2024
Chemical kinetics (redirect from Reaction kinetics)
also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different...
24 KB (3,329 words) - 05:27, 10 July 2024
and reaction conditions. Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Some reactions produce...
66 KB (8,031 words) - 09:58, 12 July 2024
Transition state theory (redirect from Absolute reaction rate theory)
chemistry, transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium...
41 KB (5,817 words) - 07:25, 23 July 2024
adjustable rate, on-demand. Nuclear chain reactions in fissionable materials produce induced nuclear fission. Various nuclear fusion reactions of light...
20 KB (2,391 words) - 17:40, 25 June 2024
the intermediate. Instead, the rate equation may be more accurately described using steady-state kinetics. The reaction involves a carbocation intermediate...
15 KB (1,970 words) - 13:54, 26 August 2024
Nucleophilic substitution (redirect from Nucleophilic substitution reaction)
affect the reaction rate of SN1 reactions. Instead of having two concentrations that affect the reaction rate, there is only one, substrate. The rate equation...
12 KB (1,415 words) - 13:08, 26 August 2024
applications. This reaction is accepted as a click chemistry reaction given the reactions' high yield, stereoselectivity, high rate, and thermodynamic...
24 KB (2,808 words) - 12:52, 24 February 2024
repeated, and equals the overall reaction rate divided by the initiation rate. Some chain reactions have complex rate equations with fractional order or...
18 KB (2,580 words) - 11:04, 9 August 2024
involved in the rate-determining step. What distinguishes SN2 from the other major type of nucleophilic substitution, the SN1 reaction, is that the displacement...
21 KB (2,554 words) - 05:01, 13 September 2024
the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the...
21 KB (2,788 words) - 21:20, 24 April 2024
Ozone–oxygen cycle (redirect from Chapman reaction)
so: (rate of reaction 2) = (rate of reaction 3) + (rate of reaction 4). It thus follows that: (rate of reaction 2) + (rate of reaction 5) = (rate of reaction...
14 KB (1,795 words) - 19:03, 1 April 2024
The erythrocyte sedimentation rate (ESR or sed rate) is the rate at which red blood cells in anticoagulated whole blood descend in a standardized tube...
25 KB (2,814 words) - 17:29, 8 August 2024
Catalysis (redirect from Catalytic reaction)
increase in rate of a chemical reaction due to an added substance known as a catalyst (/ˈkætəlɪst/). Catalysts are not consumed by the reaction and remain...
46 KB (5,235 words) - 10:42, 26 September 2024
three-dimensional structure. Like all catalysts, enzymes increase the reaction rate by lowering its activation energy. Some enzymes can make their conversion...
96 KB (9,819 words) - 12:03, 26 July 2024
Nuclear fusion (redirect from Thermonuclear reaction)
Nuclear fusion is a reaction in which two or more atomic nuclei, usually deuterium and tritium (hydrogen isotopes), combine to form one or more different...
97 KB (10,348 words) - 21:34, 20 September 2024
is an equation in electrochemical kinetics relating the rate of an electrochemical reaction to the overpotential. The Tafel equation was first deduced...
10 KB (1,171 words) - 15:02, 29 June 2024
Stellar nucleosynthesis (section Reaction rate)
and later by Edward Teller and Gamow himself to derive the rate at which nuclear reactions would occur at the high temperatures believed to exist in stellar...
37 KB (4,238 words) - 13:52, 22 September 2024
Chemical equilibrium (redirect from Equilibrium reaction)
when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not...
44 KB (6,697 words) - 10:34, 21 September 2024
overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting...
12 KB (1,736 words) - 20:02, 2 September 2024
(often with respect to time) Rate function, a function used to quantify the probabilities of a rare event Reaction rate, in chemistry the speed at which...
1 KB (242 words) - 04:34, 13 March 2023
chemistry, the law of mass action is the proposition that the rate of a chemical reaction is directly proportional to the product of the activities or...
27 KB (3,433 words) - 11:13, 29 June 2024
Half-life (redirect from Reaction half life)
for a zero order reaction depends on the initial concentration and the rate constant. In first order reactions, the rate of reaction will be proportional...
17 KB (2,182 words) - 21:20, 12 August 2024
Steady state (chemistry) (section Reaction rates)
system at chemical equilibrium, the net reaction rate is zero (products transform into reactants at the same rate as reactants transform into products)...
14 KB (2,843 words) - 12:24, 10 August 2024
on both sides of the reaction, we would be left with the original reaction.) When determining the overall rate law for a reaction, the slowest step is...
13 KB (1,686 words) - 06:29, 9 August 2024
conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power of a nuclear power plant. The...
22 KB (2,865 words) - 21:03, 11 August 2024
Fenton's reagent (redirect from Fenton reaction)
reaction performance. Thus ongoing research has been done to optimize pH and amongst other parameters for greater reaction rates. The Fenton reaction...
16 KB (1,631 words) - 05:50, 11 September 2024