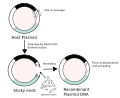
In food safety, the concept of substantial equivalence holds that the safety of a new food, particularly one that has been genetically modified (GM),...
17 KB (1,789 words) - 19:44, 5 November 2024
Countries such as the United States, Canada, Lebanon and Egypt use substantial equivalence as the starting point when assessing safety, while many countries...
78 KB (8,559 words) - 09:58, 17 November 2024
replace the substantial equivalence standard with safety studies. In a 2016 review, Domingo criticized the use of the "substantial equivalence" concept as...
307 KB (32,799 words) - 05:41, 17 November 2024
operations (Decision BS-I/3, SCBD 2004). Biosafety Clearing-House Substantial equivalence Nagoya Protocol, another supplementary protocol adopted by the...
26 KB (2,444 words) - 04:34, 10 April 2024
only looks at verifiable scientific risks and uses the concept of substantial equivalence. The European Union by contrast has possibly the most stringent...
134 KB (14,252 words) - 05:41, 17 November 2024
traditional crops. Moreover, they believe that the FDA follows the substantial equivalence rule not because of the science, but because the FDA was corrupted...
29 KB (3,428 words) - 19:15, 30 October 2024
existed prior to February 15, 2007, applicants can apply via the Substantial Equivalence (SE) regulatory pathway. On May 10, 2016, the US FDA finalized...
4 KB (385 words) - 14:26, 10 June 2024
United States. The Washington Accord recognizes that there is substantial equivalence of programs accredited by those signatories. Graduates of accredited...
7 KB (322 words) - 12:28, 18 November 2024
Organization, Geneva WHO (1995) Application of the principle of substantial equivalence to the safety evaluation of foods or food components from plants...
59 KB (6,419 words) - 05:18, 5 September 2024
controversial. Cultivation of herbicide-resistant corn in the EU provides substantial farm-level benefits. Bt maize/Bt corn is a variant of maize that has...
77 KB (8,544 words) - 05:41, 17 November 2024
responsible for β-carotene biosynthesis. This variety of rice holds substantial promise for reducing the incidence of vitamin A deficiency in the world's...
35 KB (3,892 words) - 13:12, 15 November 2024
Countries such as the United States, Canada, Lebanon and Egypt use substantial equivalence to determine if further testing is required, while many countries...
144 KB (15,863 words) - 05:40, 17 November 2024
Rice Soybean Potato History and regulation History Regulation Substantial equivalence Cartagena Protocol on Biosafety Process Techniques Molecular cloning...
13 KB (1,362 words) - 02:47, 22 May 2024
looks at verifiable scientific risks and uses the concept of substantial equivalence. Whether gene edited organisms should be regulated the same as...
223 KB (24,627 words) - 12:34, 17 November 2024
in their legally prescribed duty to enforce the requirement of "substantial equivalence" in Hasidic schools, though not naming specific schools of concern...
60 KB (5,592 words) - 19:08, 15 October 2024
Rice Soybean Potato History and regulation History Regulation Substantial equivalence Cartagena Protocol on Biosafety Process Techniques Molecular cloning...
25 KB (2,878 words) - 19:30, 14 August 2024
Rice Soybean Potato History and regulation History Regulation Substantial equivalence Cartagena Protocol on Biosafety Process Techniques Molecular cloning...
102 KB (11,029 words) - 03:36, 21 November 2024
Rice Soybean Potato History and regulation History Regulation Substantial equivalence Cartagena Protocol on Biosafety Process Techniques Molecular cloning...
120 KB (12,426 words) - 20:22, 14 November 2024
(26), 2005 PDF document. "Section 905(j) Reports: Demonstrating Substantial Equivalence for Tobacco Products" (PDF). Food and Drug Administration. January...
14 KB (1,512 words) - 15:54, 13 September 2024
Accord, signed by the Engineering Council in 1989, recognises "substantial equivalence" between the academic requirements for registration between signatories...
17 KB (1,848 words) - 23:16, 26 September 2024
Rice Soybean Potato History and regulation History Regulation Substantial equivalence Cartagena Protocol on Biosafety Process Techniques Molecular cloning...
27 KB (3,148 words) - 19:42, 31 October 2024
ingredient. Generally recognized as safe and effective Novel food Substantial equivalence "How U.S. FDA's GRAS Notification Program Works (original January...
10 KB (1,097 words) - 23:32, 15 October 2023
Rice Soybean Potato History and regulation History Regulation Substantial equivalence Cartagena Protocol on Biosafety Process Techniques Molecular cloning...
24 KB (2,659 words) - 09:07, 26 September 2024
2008 to 2012. They found that scientific evidence supporting "substantial equivalence" to other devices already on the market was required by law to...
79 KB (8,864 words) - 13:29, 22 November 2024
Rice Soybean Potato History and regulation History Regulation Substantial equivalence Cartagena Protocol on Biosafety Process Techniques Molecular cloning...
24 KB (2,559 words) - 09:20, 23 February 2024
health and safety.[citation needed] Generally recognized as safe Substantial equivalence Rajasekaran, A.; Kalaivani, M. (22 May 2012). "Designer foods and...
15 KB (1,856 words) - 08:17, 17 October 2024
conclude that the data presented in these articles does not provide any substantial evidence of GMO harm. The presented articles suggesting possible harm...
43 KB (4,918 words) - 05:41, 17 November 2024
Rice Soybean Potato History and regulation History Regulation Substantial equivalence Cartagena Protocol on Biosafety Process Techniques Molecular cloning...
55 KB (6,765 words) - 09:23, 25 May 2024
Rice Soybean Potato History and regulation History Regulation Substantial equivalence Cartagena Protocol on Biosafety Process Techniques Molecular cloning...
71 KB (8,294 words) - 16:50, 27 September 2024
sources. In each case, these applications have been simplified or substantial equivalence applications, because astaxanthin is recognised as a food component...
29 KB (2,687 words) - 17:43, 13 October 2024