Pfizer–BioNTech COVID-19 vaccine (redirect from Comirnaty)
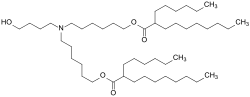
The Pfizer–BioNTech COVID-19 vaccine, sold under the brand name Comirnaty, is an mRNA-based COVID-19 vaccine developed by the German biotechnology company...
260 KB (22,097 words) - 23:43, 14 October 2024
name Comirnaty, is an mRNA vaccine produced by the German company BioNTech and the American company Pfizer. In Hong Kong, Macau, and Taiwan, Comirnaty is...
322 KB (19,558 words) - 03:33, 16 September 2024
Pfizer–BioNTech COVID-19 vaccine codenamed BNT162b2 (later branded as Comirnaty). This approval enabled the start of the UK's COVID-19 vaccination programme...
26 KB (2,421 words) - 21:40, 9 November 2024
Kingdom, 138,000 people had received the Pfizer-BioNTech COVID‑19 vaccine Comirnaty by 16 December, during the first week of the UK vaccination programme...
122 KB (11,033 words) - 06:12, 17 November 2024
vaccines, of which the first authorized were COVID-19 vaccines (such as Comirnaty and Spikevax). mRNA is produced by synthesising a ribonucleic acid (RNA)...
24 KB (2,503 words) - 15:45, 11 November 2024
Erstens stimmt es nicht, dass die Zusatzstoffe unerlaubt sind. Denn Comirnaty ist zugelassen und damit sind auch alle darin enthaltenen Hilfsstoffe...
8 KB (782 words) - 12:58, 24 January 2024
is the first RNAi drug to receive regulatory approval. The second is Comirnaty, the COVID-19 mRNA vaccine developed by Pfizer/BioNTech that has received...
19 KB (1,222 words) - 20:56, 1 May 2024
do not jeopardize the quality and integrity of the data from the main Comirnaty trial and have no impact on the benefit-risk assessment or on the conclusions...
168 KB (14,575 words) - 20:40, 12 November 2024
$2 billion NxStage acquisition". Modern Healthcare. "Package Insert - Comirnaty". U.S. Food and Drug Administration. December 2021. Retrieved 2022-05-24...
19 KB (1,666 words) - 12:24, 14 September 2024
593–603. doi:10.1016/j.drudis.2020.11.025. PMC 7694556. PMID 33253920. "Comirnaty and Pfizer-BioNTech COVID-19 Vaccine". FDA. January 3, 2022. Archived...
121 KB (12,011 words) - 17:43, 12 November 2024
Pharma Proprietary medications Pfizer Viagra (sildenafil), Comirnaty (tozinameran, co-op with BioNTech) GlaxoSmithKline Amoxil (amoxicillin), Ventolin...
41 KB (3,595 words) - 21:42, 3 November 2024
December 2020. "EMA recommends standard marketing authorizations for Comirnaty and Spikevax COVID-19 vaccines". European Medicines Agency. 16 September...
202 KB (23,039 words) - 16:40, 13 November 2024
Hospitalization effectiveness Mortality effectiveness Spikevax 91.45% 78% 93.46% Comirnaty 80.34% 84.26% 89.83% Sputnik V 78.75% 81.38% 87.7% Covishield 80.79% 80...
154 KB (13,917 words) - 19:29, 18 November 2024
recommendations for the rollout of the vaccine, recommending that the Pfizer Comirnaty vaccine be used for people aged under 60 years if the person has not already...
214 KB (17,080 words) - 15:51, 27 September 2024
Tozinameran – COVID-19 vaccine from Pfizer BioNTech, sold under the brand name Comirnaty Uğur Şahin – German oncologist and immunologist (born 1965), co-founder...
53 KB (5,090 words) - 14:57, 6 November 2024
for Pfizer and BioNTech's COVID-19 vaccine, Comirnaty, and its nonproprietary name, tozinameran. Comirnaty was the first COVID-19 vaccine brand name approved...
10 KB (856 words) - 13:10, 28 October 2024
Medicine), showed that a third dose of the Comirnaty vaccine given to those who received two doses of either Comirnaty or CoronaVac provided protective levels...
121 KB (11,165 words) - 08:08, 24 September 2024
from Pfizer". ICU Medical. Retrieved 28 April 2017. "Package Insert - Comirnaty". U.S. Food and Drug Administration. December 2021. Retrieved 2022-05-24...
17 KB (1,414 words) - 23:00, 21 October 2024
available in the U.S. is not FDA-approved" because it was not the "Comirnaty version". "Comirnaty" is the United States Adopted Name that was assigned to the...
112 KB (9,790 words) - 19:00, 18 November 2024
distribution began, but was assigned the United States Adopted Name "Comirnaty" upon its approval. Some anti-vaccine advocates have made claims surrounding...
132 KB (12,911 words) - 03:13, 18 November 2024
currently in use; following approval of the Pfizer–BioNTech COVID-19 vaccine (Comirnaty), vaccines developed by University of Oxford and AstraZeneca (Vaxzevria)...
118 KB (9,094 words) - 19:26, 12 September 2024
cooperation with the American company Pfizer sold under the brand name Comirnaty. On 24 August 2020 WHO stated that COVAX had nine CEPI-supported vaccine...
57 KB (4,064 words) - 08:20, 8 November 2024
injection" (PDF). Regulation 174. MHRA. 15 December 2020. Assessment report, Comirnaty, Common name: COVID-19 mRNA vaccine (nucleoside-modified); Procedure No...
3 KB (205 words) - 21:13, 14 November 2024
(CHMP) (19 February 2021). "European public assessment report (EPAR) – Comirnaty, INN-COVID-19 mRNA Vaccine (nucleoside-modified)" (PDF). European Medicines...
43 KB (3,701 words) - 20:03, 12 September 2024
month. On 26 August 2021, Pfizer Inc. and BioNTech, the developers of the COMIRNATY vaccine against COVID-19, announced a partnership with Eurofarma to manufacture...
8 KB (733 words) - 21:16, 8 July 2024
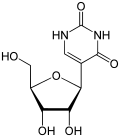
vaccine from BioNTech/Pfizer, also known as BNT162b2, tozinameran or Comirnaty, all U's have been substituted with N1-methylpseudouridine, a nucleoside...
25 KB (2,895 words) - 15:43, 8 November 2024
president Donald Trump to emphasize that the pandemic started in China. Comirnaty The commercial name for the FDA approved COVID-19 vaccine from Pfizer...
8 KB (853 words) - 22:01, 31 May 2024
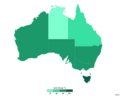
provisionally approved the two-dose Pfizer–BioNTech COVID-19 vaccine, named COMIRNATY, for use within Australia. The provisional approval only recommends the...
31 KB (2,811 words) - 08:32, 16 November 2024
Advisory Group on Immunisation (ATAGI) recommended that the Pfizer vaccine (Comirnaty) be preferred over the AstraZeneca vaccine in people under the age of...
177 KB (12,119 words) - 04:28, 6 October 2024
10 January 2022. "EMA recommends standard marketing authorisations for Comirnaty and Spikevax COVID-19 vaccines". European Medicines Agency (Press release)...
177 KB (13,698 words) - 16:52, 23 October 2024