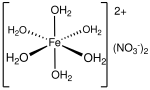
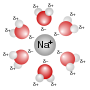
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species...
14 KB (1,508 words) - 01:13, 4 June 2023
Some of the simplest members of such complexes are described in metal aquo complexes, metal ammine complexes, Examples: [Co(EDTA)]−, [Co(NH3)6]3+, [Fe(C2O4)3]3-...
57 KB (5,554 words) - 03:54, 13 August 2024
water in metal aquo complexes to methyl groups as in tetraethyl lead. Usually metal complexes consist of a mixture of ligands. Toxic metal complexes can be...
21 KB (2,417 words) - 04:38, 31 July 2024
than water, metal ammine complexes are stabilized relative to the corresponding aquo complexes. For similar reasons, metal ammine complexes are less strongly...
16 KB (1,787 words) - 12:15, 19 July 2024
oxygens and the protons of aquo ligands. Stoichiometrically simple complexes are often multimetallic. One family are the basic metal acetates, of the stoichiometry...
13 KB (1,361 words) - 23:10, 21 July 2024
hydrates. The nitrate anion often binds to the metal, especially for those salts with fewer than six aquo ligands. Nitrates are uncommon in nature, so few...
49 KB (2,952 words) - 16:33, 4 August 2024
bromopentaamminecobalt(III) undergoes the following aquation reaction to give a metal aquo complex: [Co(NH3)5Br]2+ + H2O → [Co(NH3)5(H2O)]3+ + Br− This aquation reaction...
1 KB (152 words) - 02:47, 28 December 2023
commonly encountered as the green hexahydrate, Fe(NO3)2·6H2O, which is a metal aquo complex, however it is not commercially available unlike iron(III) nitrate...
8 KB (733 words) - 10:24, 18 August 2024
Hydroxide (section Alkali metals)
derivatives. Many can be made by deprotonation of the corresponding metal aquo complex. LnM(OH2) + B ⇌ LnM(OH) + BH+ (L = ligand, B = base) Vanadic acid...
41 KB (4,896 words) - 02:07, 5 July 2024
Ferrous (redirect from Ferrous metal)
to the more soluble iron(II).) In contrast to iron(III) aquo complexes, iron(II) aquo complexes are soluble in water near neutral pH.[citation needed]...
9 KB (968 words) - 13:19, 15 April 2024
Hydrolysis (section Metal aqua ions)
contribute to carcinogenesis and ageing. Metal ions are Lewis acids, and in aqueous solution they form metal aquo complexes of the general formula M(H2O)nm+....
18 KB (2,269 words) - 02:00, 19 June 2024
Bioinorganic chemistry (redirect from Metal metabolism)
these are metalloproteins. Often the reacting water is a ligand (see metal aquo complex). Examples of hydrolase enzymes are carbonic anhydrase, metallophosphatases...
21 KB (2,364 words) - 01:44, 14 August 2024
Octahedral molecular geometry (redirect from Octahedral complex)
presence of acid or base. Addition of concentrated HCl converts the aquo complex back to the chloride, via an anation process. Octahedral clusters AXE...
15 KB (1,554 words) - 05:25, 31 October 2023
Copper (redirect from Copper sheet metal)
(speed of water ligands attaching and detaching) for any transition metal aquo complex. Adding aqueous sodium hydroxide causes the precipitation of light...
121 KB (13,773 words) - 19:46, 16 August 2024
character makes it a common ligand in transition metal complexes, examples of which include metal aquo complexes such as Fe(H 2O)2+ 6 to perrhenic acid, which...
88 KB (9,550 words) - 13:45, 9 August 2024
sulfate behave identically. These aqueous solutions consist of the metal aquo complex [Zn(H2O)6]2+ and SO2− 4 ions. Barium sulfate forms when these solutions...
14 KB (1,152 words) - 03:40, 26 July 2024
material. All the iron(II) sulfates dissolve in water to give the same aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry and is paramagnetic...
25 KB (1,843 words) - 17:40, 30 June 2024
Copper compounds (section Cu–O and Cu–N complexes)
(speed of water ligands attaching and detaching) for any transition metal aquo complex. Adding aqueous sodium hydroxide causes the precipitation of light...
14 KB (1,500 words) - 21:26, 19 August 2024
A transition metal nitrate complex is a coordination compound containing one or more nitrate ligands. Such complexes are common starting reagents for...
11 KB (1,122 words) - 18:54, 20 November 2023
Salicylaldoxime (section Extraction of metals)
metal aquo complex and aldoxime are liberated. In this way the ligand is used as a recyclable extractant. It typically forms charge-neutral complexes...
4 KB (377 words) - 20:19, 11 February 2023
Nickel (category Transition metals)
forms the metal aquo complex [Ni(H2O)6]2+. Dehydration of NiCl2·6H2O gives yellow anhydrous NiCl2. Some tetracoordinate nickel(II) complexes, e.g....
89 KB (9,740 words) - 09:36, 21 June 2024
other amino acids. Commonly amino acid complexes are prepared by ligand displacement reactions of metal aquo complexes and the conjugate bases of amino acids:...
10 KB (1,148 words) - 19:56, 9 February 2024
Cobalt (category Transition metals)
simple compounds is +2 (cobalt(II)). These salts form the pink-colored metal aquo complex [Co(H 2O) 6]2+ in water. Addition of chloride gives the intensely...
96 KB (10,294 words) - 12:45, 13 August 2024
which metal ions form polymeric oxides in aqueous solution. The phenomenon is important for understanding the relationship between metal aquo complexes and...
5 KB (722 words) - 09:00, 19 February 2024
tetrahedral anions and cations as well their interactions with the metal aquo complex [M(H2O)6]2+. Perhaps the best-known is Mohr's salt, ferrous ammonium...
41 KB (2,957 words) - 00:37, 19 March 2024
Sodium chloride (category Alkali metal chlorides)
become surrounded by polar water molecules. These solutions consist of metal aquo complex with the formula [Na(H2O)8]+, with the Na–O distance of 250 pm. The...
32 KB (3,459 words) - 12:15, 7 August 2024
and cations from a dissolved salt in a solvent. Metal ions in aqueous solutions form metal aquo complexes. This number can be determined by various methods...
5 KB (642 words) - 09:58, 7 January 2024
Aluminium chloride (category Metal halides)
[Al(H2O)6]Cl3 → Al(OH)3 + 3 HCl + 3 H2O Like metal aquo complexes, aqueous AlCl3 is acidic owing to the ionization of the aquo ligands: [Al(H2O)6]3+ ⇌ [Al(OH)(H2O)5]2+...
23 KB (1,721 words) - 09:46, 18 July 2024
CoSO4(H2O) + 5 H2O CoSO4(H2O) → CoSO4 + H2O The hexahydrate is a metal aquo complex consisting of octahedral [Co(H2O)6]2+ ions associated with sulfate...
10 KB (735 words) - 21:40, 1 July 2024
ubiquitous. In all metal aquo-complexes [M(H2O)n]m+, the bonding between water and the metal cation is described as a coordinate covalent bond. Metal-ligand interactions...
10 KB (1,313 words) - 13:10, 17 August 2024