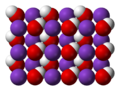
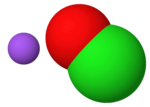
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of...
54 KB (5,925 words) - 15:03, 14 November 2024
catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton...
41 KB (4,891 words) - 13:03, 26 August 2024
Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide...
69 KB (8,043 words) - 01:28, 30 October 2024
Potassium hydroxide is an inorganic compound with the formula KOH, and is commonly called caustic potash. Along with sodium hydroxide (NaOH), KOH is a...
21 KB (2,063 words) - 06:28, 7 October 2024
Lye (category Hydroxides)
A lye refers to sodium hydroxide and potassium hydroxide. The word lye most accurately refers to sodium hydroxide (NaOH),[citation needed] but historically...
13 KB (1,434 words) - 21:22, 9 November 2024
nitrogen trichloride. With excess ammonia and sodium hydroxide, hydrazine may be generated. Anhydrous sodium hypochlorite can be prepared but, like many...
55 KB (5,830 words) - 22:52, 13 November 2024
carbonating sodium hydroxide which is made using the chloralkali process. Sodium carbonate is obtained as three hydrates and as the anhydrous salt: sodium carbonate...
32 KB (3,130 words) - 02:46, 8 November 2024
Base (chemistry) (section Alkalinity of non-hydroxides)
alcohol. When dissolved in water, the strong base sodium hydroxide ionizes into hydroxide and sodium ions: NaOH ⟶ Na + + OH − {\displaystyle {\ce {NaOH...
26 KB (3,041 words) - 06:18, 8 August 2024
bauxite in sodium hydroxide at temperatures up to 270 °C (518 °F). The waste solid, bauxite tailings, is removed and aluminium hydroxide is precipitated...
24 KB (2,174 words) - 07:32, 23 October 2024
for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide (caustic soda), which are...
16 KB (1,818 words) - 10:16, 14 November 2024
hydroxide will dissolve because the ion is normally surrounded by water ligands; when excess sodium hydroxide is added to the solution the hydroxide ions...
4 KB (348 words) - 10:30, 27 August 2024
ions. Sodium oxide is produced by the reaction of sodium with sodium hydroxide, sodium peroxide, or sodium nitrite: 2 NaOH + 2 Na → 2 Na2O + H2 To the extent...
7 KB (542 words) - 05:02, 12 September 2024
3 + OH− Sodium bicarbonate can sometimes be used as a mild neutralization agent and a safer alternative to strong bases like sodium hydroxide. Reaction...
56 KB (5,380 words) - 08:53, 12 November 2024
where it is an intermediate in the reaction in the production of sodium hydroxide. This conversion is part of the causticizing step in the Kraft process...
22 KB (2,126 words) - 09:10, 9 November 2024
Sodium aluminate is an inorganic chemical that is used as an effective source of aluminium hydroxide for many industrial and technical applications. Pure...
7 KB (648 words) - 09:38, 11 April 2024
Sodium tallowate is made from sodium hydroxide (a.k.a., caustic soda, or, lye); steam; and, animal (often, cattle) fat (i.e., tallow). This process, called...
832 bytes (80 words) - 18:40, 5 October 2024
processes because potassium hydroxide and sodium hydroxide can perform nearly all the same functions of rubidium hydroxide. Metal oxide catalysts are sometimes...
3 KB (162 words) - 21:17, 14 January 2024
salts and alcohols by the action of aqueous alkali. Typically aqueous sodium hydroxide solutions are used. It is an important type of alkaline hydrolysis...
12 KB (1,246 words) - 13:38, 28 January 2024
chloralkali process, the industrial process to produce chlorine and sodium hydroxide, according to the chemical equation 2 NaCl + 2 H 2 O → e l e c t r...
32 KB (3,460 words) - 22:33, 12 November 2024
Flavonoid (redirect from Sodium hydroxide test)
compound is dissolved in water, warmed, and filtered. 10% aqueous sodium hydroxide is added to 2 ml of this solution. This produces a yellow coloration...
34 KB (3,268 words) - 18:08, 30 September 2024
hydroxides such as sodium hydroxide and potassium hydroxide. It is the strongest of the five alkali metal hydroxides. Fused Caesium hydroxide dissolves glass...
6 KB (380 words) - 23:33, 18 March 2024
solution known as vinegar, with sodium carbonate ("washing soda"), sodium bicarbonate ("baking soda"), or sodium hydroxide ("lye", or "caustic soda"). Any...
16 KB (1,145 words) - 00:41, 26 October 2024
the half-neutralization of hydrogen sulfide (H2S) with sodium hydroxide (NaOH). NaSH and sodium sulfide are used industrially, often for similar purposes...
6 KB (432 words) - 14:05, 24 January 2024
Carbon dioxide scrubber (section Sodium hydroxide)
kJ/mol Other strong bases such as soda lime, sodium hydroxide, potassium hydroxide, and lithium hydroxide are able to remove carbon dioxide by chemically...
15 KB (1,746 words) - 00:45, 6 August 2024
formate is produced by absorbing carbon monoxide under pressure in solid sodium hydroxide at 130 °C and 6-8 bar pressure: CO + NaOH → HCO2Na Because of the low-cost...
10 KB (1,044 words) - 21:41, 12 March 2024
has several uses. It can be prepared by reacting a sodium-containing base such as sodium hydroxide with iodic acid, for example: HIO3 + NaOH → NaIO3 +...
5 KB (228 words) - 14:09, 24 January 2024
Soda lime (category Hydroxides)
Soda lime, a mixture of sodium hydroxide (NaOH) and calcium oxide (CaO), is used in granular form within recirculating breathing environments like general...
12 KB (1,310 words) - 16:00, 4 October 2024
The production of chlorine results in the co-products caustic soda (sodium hydroxide, NaOH) and hydrogen gas (H2). These two products, as well as chlorine...
22 KB (2,501 words) - 12:32, 29 April 2024
acids with sodium hydroxide. Sodium salts can be categorized into: sodium salts of carboxylic acids (e. g. sodium formate, HCOONa, the sodium salt of formic...
3 KB (314 words) - 00:05, 18 October 2024
Lithium hydroxide (LiOH) Sodium hydroxide (NaOH) Potassium hydroxide (KOH) Rubidium hydroxide (RbOH) Caesium hydroxide (CsOH) Francium hydroxide (FrOH)...
3 KB (363 words) - 13:40, 16 July 2024