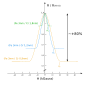
Spin states when describing transition metal coordination complexes refers to the potential spin configurations of the central metal's d electrons. For...
11 KB (1,351 words) - 17:01, 18 July 2024
Atomic orbital (redirect from Spin-orbital)
a maximum of two electrons, each with its own projection of spin m s {\displaystyle m_{s}} . The simple names s orbital, p orbital, d orbital, and f orbital...
84 KB (10,932 words) - 21:38, 12 July 2024
photons, and as spinors and bispinors for other particles such as electrons. Spinors and bispinors behave similarly to vectors: they have definite magnitudes...
72 KB (10,551 words) - 13:52, 19 July 2024
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The...
48 KB (6,597 words) - 05:32, 13 July 2024
of electrons or an arrangement of electrons in which there is an unequal number of "spin-up" and "spin-down" orientations. These atoms or electrons are...
22 KB (2,959 words) - 08:55, 31 May 2024
Spin State may refer to: Spin quantum number, a quantum number Spin states (d electrons), the potentials for high-spin and low-spin configurations of...
300 bytes (71 words) - 16:30, 12 April 2010
fermions, no two electrons can occupy the same quantum state, per the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties...
153 KB (15,428 words) - 02:28, 20 June 2024
spintronics, spin–orbit effects for electrons in semiconductors and other materials are explored for technological applications. The spin–orbit interaction...
28 KB (4,302 words) - 23:55, 25 June 2024
an electron to lie outside of the d shell. The electrons outside a d shell are the electrons which have higher energy than the electrons within the d shell...
6 KB (539 words) - 02:58, 21 January 2024
superconductor: A proposed state in which electrons are not bound as Cooper pairs but as quadruplets of electrons. Superfluid: A phase achieved by a few...
14 KB (1,718 words) - 11:24, 29 June 2024
Spintronics (redirect from Spin computing)
manipulation of a spin-polarized population of electrons, resulting in an excess of spin up or spin down electrons. The polarization of any spin dependent property...
30 KB (3,321 words) - 05:10, 25 May 2024
Paramagnetism (section d and f electrons)
their spin, unpaired electrons have a magnetic dipole moment and act like tiny magnets. An external magnetic field causes the electrons' spins to align...
28 KB (4,336 words) - 02:21, 27 March 2024
Transition metal (redirect from D-block element)
one or more unpaired d electrons. In octahedral complexes with between four and seven d electrons both high spin and low spin states are possible. Tetrahedral...
40 KB (4,504 words) - 07:15, 17 February 2024
Angular momentum coupling (redirect from Spin-spin coupling)
However, if we switch on the electron–electron interaction that depends on the distance d(1,2) between the electrons, then only a simultaneous and equal...
17 KB (2,228 words) - 20:09, 21 February 2024
quantum-mechanical nature of electrons. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. In...
60 KB (6,147 words) - 22:00, 18 May 2024
building quantum computers with electrons on helium by Platzman and Dykman in 1999. The electrostrictive binding of electrons to the surface of liquid helium...
21 KB (2,406 words) - 22:08, 10 April 2024
Crystal field theory (redirect from High spin complex)
electrons in the same orbital repel each other. So, one electron is put into each of the five d-orbitals in accord with Hund's rule, and "high spin"...
17 KB (1,998 words) - 17:53, 20 January 2024
the interaction between the magnetic moments associated with electron spin and the electrons' orbital angular momentum. Hyperfine structure, with energy...
28 KB (4,377 words) - 02:23, 18 July 2024
electron resulting from its intrinsic properties of spin and electric charge. The value of the electron magnetic moment (symbol μe) is −9.2847646917(29)×10−24 J⋅T−1...
23 KB (3,355 words) - 20:00, 11 July 2024
crystalline structure an odd number of electrons per atom results in a half-filled top band; there are free electrons at the Fermi level resulting in a metal...
36 KB (5,578 words) - 08:28, 18 March 2024
radical-pair electrons both have spin, short for spin angular momentum, which gives each separate radical a magnetic moment. Therefore, spin states can be altered...
7 KB (825 words) - 02:51, 11 December 2023
ESR dating. Electron spin resonance dating can be described as trapped charge dating. Radioactivity causes negatively charged electrons to move from...
13 KB (1,529 words) - 04:39, 13 May 2024
Singlet state (redirect from Spin singlet)
electrons are paired. The term 'singlet' originally meant a linked set of particles whose net angular momentum is zero, that is, whose overall spin quantum...
11 KB (1,647 words) - 21:09, 8 July 2024
explained if the electrons in an atom were connected or clustered in some manner. Groups of electrons were thought to occupy a set of electron shells around...
25 KB (3,449 words) - 09:07, 6 April 2024
can in principle hold up to 2(n2) electrons. For an explanation of why electrons exist in these shells, see electron configuration. Each shell consists...
28 KB (2,782 words) - 22:45, 4 May 2024
only take 2 electrons, the n = 2 shell possesses an s and a p subshell and can take 8 electrons overall, the n = 3 shell possesses s, p, and d subshells...
19 KB (2,121 words) - 15:28, 24 April 2024
minority-spin electrons the sp and d bands are hybridized, and the Fermi level lies within the d band. The hybridized spd band has a high density of states, which...
49 KB (5,642 words) - 20:27, 20 April 2024
Hund's rules (section Excited states)
{\displaystyle S} is the total spin angular momentum for all electrons. The multiplicity is also equal to the number of unpaired electrons plus one. Therefore,...
12 KB (1,584 words) - 00:34, 17 April 2024
due to spin-orbit coupling, a magnetic field pointing upwards for spin-up electrons and a magnetic field pointing downwards for spin-down electrons. The...
10 KB (1,391 words) - 18:22, 10 June 2024
defined as the total electron density of electrons of one spin minus the total electron density of the electrons of the other spin. One of the ways to...
16 KB (2,301 words) - 07:52, 1 May 2024