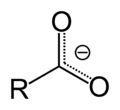
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They...
10 KB (1,008 words) - 21:11, 11 December 2024
A transition metal alkoxide complex is a kind of coordination complex containing one or more alkoxide ligands, written as RO−, where R is the organic substituent...
10 KB (907 words) - 14:05, 2 April 2024
Silicon alkoxides are a group of alkoxides, chemical compounds of silicon and an alcohol, with the formula Si(OR)4. Silicon alkoxides are important precursors...
555 bytes (43 words) - 20:52, 22 February 2023
produced from epoxides. Nucleophilic displacement of alkyl halides by alkoxides R–ONa + R′–X → R–O–R′ + NaX This reaction, the Williamson ether synthesis...
19 KB (1,843 words) - 18:54, 16 December 2024
an anhydrous base to give an ester. Catalysts are aluminium alkoxides or sodium alkoxides. Benzaldehyde reacts with sodium benzyloxide (generated from...
46 KB (4,846 words) - 04:43, 20 November 2024
deprotonated alcohol (alkoxide). This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary...
11 KB (1,339 words) - 23:53, 31 August 2024
attack another carbonyl. In the final step of the reaction, the acid and alkoxide ions formed exchange a proton. In the presence of a very high concentration...
8 KB (929 words) - 01:53, 14 November 2024
conjugate sodium alkoxide base of the alcohol formed (e.g. sodium ethoxide if ethanol is formed) is often used, since the alkoxide is regenerated. In...
7 KB (627 words) - 23:49, 14 February 2024
the alkoxide generates a carboxylic acid: The alkoxide ion is a strong base so the proton is transferred from the carboxylic acid to the alkoxide ion...
12 KB (1,246 words) - 01:02, 19 December 2024
corresponding metal-hydride-alkene. β-Hydride elimination can also occur for many alkoxide complexes as well. The main requirements are that the alkyl group possess...
18 KB (1,941 words) - 19:07, 24 December 2024
charged hydrogen ion (proton), much more readily than alcohols do (into an alkoxide ion and a proton), because the carboxylate ion is stabilized by resonance...
5 KB (604 words) - 21:59, 18 August 2024
tetrahedral intermediate, which then expels an alkoxide ion. The resulting carboxylic acid quickly protonates the alkoxide ion to give a carboxylate ion and an...
3 KB (268 words) - 19:52, 31 January 2024
malonate and the alcohol, while other alkoxide salts will cause scrambling by transesterification. Only the "same" alkoxide anion as the one that one used to...
9 KB (765 words) - 17:21, 29 November 2024
various superbasic mixtures of an alkyllithium compound and a potassium alkoxide. The reagent is named after Manfred Schlosser, although he uses the term...
3 KB (372 words) - 09:06, 12 April 2020
Titanium isopropoxide (category Alkoxides)
or TTIP, is a chemical compound with the formula Ti{OCH(CH3)2}4. This alkoxide of titanium(IV) is used in organic synthesis and materials science. It...
8 KB (654 words) - 11:22, 21 April 2024
Titanium ethoxide (redirect from Titanium alkoxide)
structure is more complex than suggested by its empirical formula. Like other alkoxides of titanium(IV) and zirconium(IV), it finds used in organic synthesis...
9 KB (865 words) - 00:05, 24 February 2024
to give, after aqueous workup, a tertiary alcohol With an alcohols or alkoxides to gives the hemiketal or its conjugate base. With a diol to the ketal...
24 KB (2,895 words) - 03:58, 25 November 2024
Tantalum(V) ethoxide (redirect from Tantalum alkoxide)
readily. It is used to prepare films of tantalum(V) oxide. Tantalum(V) alkoxides typically exist as dimers with octahedral six-coordinate tantalum metal...
18 KB (1,686 words) - 11:55, 12 April 2024
bonded to H it is an alcohol. An alkoxide can refer to salts of alcohols, and they are ionic compounds containing an alkoxide ions RO−; it is a derivative...
2 KB (197 words) - 08:43, 18 December 2023
Acetylenediol (section Alkoxide derivatives)
Acetylenediol, or ethynediol, is a chemical substance with formula HO−C≡C−OH (an ynol). It is the diol of acetylene. Acetylenediol is unstable in the condensed...
8 KB (765 words) - 05:17, 25 May 2024
Niobium(V) ethoxide (redirect from Niobium alkoxide)
Metal alkoxides rarely adopt monomeric structures, and niobium(V) ethoxide is no exception. Early studies established that niobium alkoxides aggregate...
6 KB (459 words) - 10:06, 20 June 2022
NH4Cl → 1/n (NPCl2)n + 4 HCl When the chloride groups are replaced by alkoxide (RO−), a family of polymers is produced with potentially useful properties...
109 KB (12,811 words) - 13:59, 8 December 2024
alcohol/alkoxide solvating mixture must match the alkoxy components of the reacting esters to minimize the number of different products. Many alkoxides are...
9 KB (746 words) - 07:20, 9 August 2024
ketones and aldehydes to their corresponding alcohols utilizing aluminium alkoxide catalysis in the presence of a sacrificial alcohol. The advantages of the...
14 KB (1,512 words) - 20:28, 22 July 2024
neutral or positively charged. Examples of nucleophiles are hydroxide ion, alkoxides, amines and halides. This type of reaction is found mainly in aliphatic...
66 KB (8,043 words) - 01:08, 14 October 2024
(C6H5–OH), which is acidic at the hydroxyl (OH), as charge on the oxygen (alkoxide –O−) is partially delocalized into the benzene ring. Although benzylic...
21 KB (2,094 words) - 17:10, 6 December 2024
discrete particles or network polymers. Typical precursors are metal alkoxides. Sol–gel process is used to produce ceramic nanoparticles. In this chemical...
30 KB (3,801 words) - 08:05, 6 June 2024
benzilic acid rearrangement, except that an alkoxide or an amide anion is used in place of a hydroxide ion. The alkoxide used should not be easily oxidizable...
8 KB (950 words) - 07:55, 30 December 2023
strong bases such as sodium hydride or sodium they form salts called alkoxides, with the general formula RO−M+ (where R is an alkyl and M is a metal)...
35 KB (3,855 words) - 08:18, 21 November 2024
presence of an alkoxide. The reaction is named after Russian organic chemist Vyacheslav Tishchenko, who discovered that aluminium alkoxides are effective...
7 KB (664 words) - 15:24, 14 December 2024