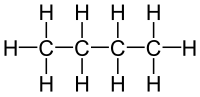
The molecular formula C4H10 (molar mass: 58.12 g/mol, exact mass: 58.0783 u) may refer to: Butane, or n-butane Isobutane, also known as methylpropane...
280 bytes (65 words) - 18:58, 22 December 2022
Butane (/ˈbjuːteɪn/) or n-butane is an alkane with the formula C4H10. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes...
23 KB (2,106 words) - 15:43, 23 June 2024
isomers of each other, since they both have the same molecular formula C4H10, but they have different structural formulas as shown. The connectivity...
24 KB (3,205 words) - 14:13, 13 July 2024
kalla den äldre C4H10, ethyl, den nyare C2H6, methyl, … " (One may then give names to ether radicals; one can call the older [one] C4H10, ethyl, the newer...
4 KB (467 words) - 12:37, 8 August 2024
reactions because of the volume of a flammable gas produced. LiC4H9 + RH → C4H10 + RLi The kinetic basicity of n-BuLi is affected by the solvent or cosolvent...
18 KB (1,856 words) - 04:22, 24 July 2024
methylcyclopropane are isomers of each other (C4H8), but are not isomers of butane (C4H10). Branched alkanes are more thermodynamically stable than their linear (or...
61 KB (5,939 words) - 18:36, 26 July 2024
or used to power the refinery's own burners. During the winter, butane (C4H10), is blended into the gasoline pool at high rates, because its high vapour...
131 KB (14,540 words) - 11:53, 9 August 2024
DTXSID1026401 InChI InChI=1S/C4H10/c1-4(2)3/h4H,1-3H3 Y Key: NNPPMTNAJDCUHE-UHFFFAOYSA-N Y SMILES CC(C)C Properties Chemical formula C4H10 Molar mass 58.124 g·mol−1...
14 KB (977 words) - 04:46, 16 August 2024
bicyclic compounds Hydrocarbons that include four atoms are: butane C4H10 isobutane C4H10 but-1-ene C4H8 but-2-ene C4H8 but-1-yne C4H6 but-2-yne C4H6 isobutylene...
2 KB (147 words) - 02:29, 28 February 2022
straight-chained alkanes begins methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), and pentane (C5H12). In that series, successive members differ in mass...
11 KB (969 words) - 19:55, 8 July 2024
Comparison of butane (C4H10) isomer boiling points Common name n-butane isobutane IUPAC name butane 2-methylpropane Molecular form Boiling point (°C)...
18 KB (2,273 words) - 23:20, 5 August 2024
Dow/Petromont gas propyne 30%, propadiene 14%, propylene 43%, propane 7%, C4H10 (isobutane, butane) 6% might be more typical. MAPP has an energy content...
8 KB (1,094 words) - 14:19, 4 July 2024
formulas for this molecule: the empirical formula C2H5 the molecular formula C4H10 and the condensed formula (or semi-structural formula) CH3CH2CH2CH3....
13 KB (1,445 words) - 06:32, 11 August 2024
gases such as ethane (C2H6), propane (C3H8), normal butane (n-C4H10), isobutane (i-C4H10), pentanes and even higher-molecular-mass hydrocarbons. The raw...
19 KB (2,263 words) - 14:13, 25 February 2024
ISBN 978-0-85404-182-4. The saturated unbranched acyclic hydrocarbons C2H6, C3H8, and C4H10 have the retained names ethane, propane, and butane, respectively. IUPAC...
30 KB (3,021 words) - 10:07, 11 August 2024
acetic acid according to the chemical equation, illustrated with butane: 2 C4H10 + 5 O2 → 4 CH3CO2H + 2 H2O Such oxidations require metal catalyst, such...
62 KB (6,567 words) - 16:13, 20 July 2024
global warming potentials of ethane (C2H6), propane (C3H8), and butane (C4H10)", Atmos. Sci. Lett., 2018, 19:e804 (2): e804, Bibcode:2018AtScL..19E.804H...
210 KB (3,317 words) - 21:06, 27 June 2024
Alkane C4H6 1,2-Butadiene Diene C4H6 1-Butyne Alkyne C4H8 1-Butene Alkene C4H10 Butane Alkane C6H10 Cyclohexene Cycloalkene C5H12 n-pentane Alkane C7H14...
5 KB (297 words) - 05:18, 11 December 2023
1 5.9% 2 C2H6 + 7 O2 → 4 CO2 + 6 H2O Butane 15.44 : 1 30.98 : 1 6.1% 2 C4H10 + 13 O2 → 8 CO2 + 10 H2O Pentane 15.31 : 1 38.13 : 1 6.1% C5H12 + 8 O2 →...
35 KB (5,275 words) - 19:20, 16 August 2024
compounds: 1-Butyne (ethylacetylene) 2-Butyne (dimethylacetylene) C4H6 Butane (C4H10) Butene (C4H8) This set index article lists chemical compounds articles...
403 bytes (68 words) - 23:09, 21 April 2024
anhydride is produced by the V2O5-catalysed oxidation of butane with air: C4H10 + 4 O2 → C2H2(CO)2O + 8 H2O Maleic anhydride is used for the production...
17 KB (1,550 words) - 08:54, 2 August 2024
Heavier gaseous hydrocarbons: propane (C3H8), normal butane (n-C4H10), isobutane (i-C4H10) and pentanes. All of these are collectively referred to as Natural...
29 KB (3,295 words) - 20:35, 24 July 2024
threonine Thr 72-19-5 C4H9Na n-Butylsodium C4H9OH butyl alcohol 71-36-3 C4H10 butane 106-97-8 2-methylpropane 75-28-5 C4H10O diethyl ether 60-29-7 C4H10O2...
182 KB (107 words) - 15:39, 24 July 2024
benzene route, whereas vanadium phosphate is used for the butane route: C4H10 + 3.5 O2 → C4H2O3 + 4 H2O ∆H = −1236 kJ/mol The main competing process entails...
16 KB (1,492 words) - 15:43, 23 November 2023
methyl methane 3 1 1 C3H8 propane dimethyl methane; propyl hydride 4 2 2 C4H10 n-butane butyl hydride; methylethyl methane 5 3 3 C5H12 n-pentane amyl hydride;...
20 KB (133 words) - 16:53, 6 October 2023
molecules of the same element O2 Dioxygen 0.0 CxAy Most hydrocarbon compounds C3H8 Propane 0.083 CxAy Hydrocarbon with center of inversion C4H10 Butane 0.0...
24 KB (2,751 words) - 06:38, 15 August 2024
Ethylene 12.5 52.5 C2H2 Acetylene 54.2 226.8 C3H8 Propane −25.0 −104.6 C4H10 n-Butane −30.0 −125.5 C5H12 n-Pentane −35.1 −146.9 C6H14 n-Hexane −40.0...
29 KB (1,880 words) - 09:26, 17 August 2024
mostly methane (CH4), along with ethane (C2H6), propane (C3H8) and butane (C4H10). Other gases also occur in natural gas, notably CO2. These gases have wide-ranging...
129 KB (13,187 words) - 19:00, 3 August 2024
T = 20 °C Ethane C2H6 9.27 T = 20 °C Propyne C3H4 8.67 T = 20 °C Propene C3H6 8.39 T = 20 °C Propane C3H8 8.18 T = 20 °C Butane C4H10 7.49 T = 20 °C...
37 KB (2,473 words) - 12:11, 7 August 2024
+ ∙ + 2 e − ( + C 3 H 7 + and other ions ) {\displaystyle {\ce {C4H10{}+e^{-}->C4H10^{+\bullet }{}+2e^{-}}}({\ce {+C3H7+}}{\text{and other ions}})} C...
16 KB (2,129 words) - 18:11, 5 June 2024