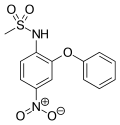
Rofecoxib is a COX-2-selective nonsteroidal anti-inflammatory drug (NSAID). It was marketed by Merck & Co. to treat osteoarthritis, rheumatoid arthritis...
76 KB (8,141 words) - 20:25, 3 August 2024
reduces the risk of peptic ulceration and is the main feature of celecoxib, rofecoxib, and other members of this drug class. After several COX-2–inhibiting...
42 KB (4,944 words) - 11:56, 4 April 2024
became the building-block for synthesis of COX-2 inhibitors. Celecoxib and rofecoxib, the first COX-2 inhibitors to reach market, were based on DuP-697. It...
37 KB (4,463 words) - 13:48, 4 June 2023
complicated upper gastrointestinal events are similar between two groups. Like rofecoxib's VIGOR trial, the MEDAL Program was also criticized, this time due to...
13 KB (1,155 words) - 14:50, 7 September 2024
heart attack. As a result, certain COX-2 selective inhibitors—such as rofecoxib—are no longer used due to the high risk of undiagnosed vascular disease...
111 KB (11,585 words) - 12:08, 31 October 2024
(traditional NSAIDs block both versions in general). These drugs (such as rofecoxib, celecoxib, and etoricoxib) are equally effective analgesics when compared...
109 KB (5,859 words) - 00:50, 28 October 2024
advice to physicians regarding celecoxib's safety. The COX-2 inhibitor rofecoxib (Vioxx) was removed from the market in 2004 due to its risk. Like all...
61 KB (6,086 words) - 21:24, 28 September 2024
the dosage and its effects are comparable to human medications such as rofecoxib and piroxicam. Grapiprant has also been tested in humans, and was researched to...
11 KB (1,033 words) - 21:58, 15 September 2024
of increased risks of heart attacks with the selective COX-2 inhibitor rofecoxib in 2004, attention has focused on all the other members of the nonsteroidal...
56 KB (5,251 words) - 06:17, 13 October 2024
double-blind, clinical trial comparing the efficacy of nimesulide, celecoxib and rofecoxib in osteoarthritis of the knee". Drugs. 63 (Suppl 1): 37–46. doi:10...
19 KB (1,789 words) - 20:47, 17 September 2024
COX-2 inhibitors Celecoxib (+tramadol) Etoricoxib Lumiracoxib ‡ Parecoxib Rofecoxib ‡ Valdecoxib ‡ Fenamates Flufenamic acid Meclofenamic acid Mefenamic acid...
116 KB (11,202 words) - 07:03, 1 November 2024
COX-2 inhibitors Celecoxib (+tramadol) Etoricoxib Lumiracoxib ‡ Parecoxib Rofecoxib ‡ Valdecoxib ‡ Fenamates Flufenamic acid Meclofenamic acid Mefenamic acid...
165 KB (17,237 words) - 23:10, 27 October 2024
Ramelteon Rasagiline Reboxetine Ribavirin Rimonabant Risperidone Rivastigmine Rofecoxib Ropinirole Rotigotine Rufinamide Selegiline Sertraline Sevoflurane Sulpiride...
26 KB (1,722 words) - 21:40, 3 October 2024
Tanaka W, et al. (October 2000). "Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1...
38 KB (3,585 words) - 09:02, 29 October 2024
A.; et al. (2000). "Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis". New England Journal...
35 KB (3,648 words) - 06:45, 6 October 2024
one of the first researchers to question the cardiovascular safety of rofecoxib (Vioxx), culminating in that drug's withdrawal from the market. Topol's...
28 KB (3,089 words) - 09:58, 27 October 2024
thromboxane unbalanced by prostacyclin (which is reduced by COX-2 inhibition). Rofecoxib (brand name Vioxx) was withdrawn in 2004 because of such concerns. Some...
16 KB (1,779 words) - 11:08, 3 July 2024
COX-2 inhibitors Celecoxib (+tramadol) Etoricoxib Lumiracoxib ‡ Parecoxib Rofecoxib ‡ Valdecoxib ‡ Fenamates Flufenamic acid Meclofenamic acid Mefenamic acid...
174 KB (16,179 words) - 22:49, 30 October 2024
effective for a small number of people. The COX-2 selective inhibitor rofecoxib was removed from the market in 2004, as cardiovascular events were associated...
131 KB (13,644 words) - 23:06, 28 October 2024
COX-2 inhibitors Celecoxib (+tramadol) Etoricoxib Lumiracoxib ‡ Parecoxib Rofecoxib ‡ Valdecoxib ‡ Fenamates Flufenamic acid Meclofenamic acid Mefenamic acid...
256 KB (26,705 words) - 22:28, 1 November 2024
COX-2 inhibitors Celecoxib (+tramadol) Etoricoxib Lumiracoxib ‡ Parecoxib Rofecoxib ‡ Valdecoxib ‡ Fenamates Flufenamic acid Meclofenamic acid Mefenamic acid...
141 KB (13,802 words) - 13:41, 27 October 2024
caution in those with gastrointestinal, cardiovascular, or kidney problems. Rofecoxib was withdrawn from the global market as its long-term use was associated...
148 KB (16,082 words) - 23:08, 18 October 2024
COX-2 inhibitors Celecoxib (+tramadol) Etoricoxib Lumiracoxib ‡ Parecoxib Rofecoxib ‡ Valdecoxib ‡ Fenamates Flufenamic acid Meclofenamic acid Mefenamic acid...
116 KB (11,944 words) - 17:09, 1 November 2024
COX-2 inhibitors Celecoxib (+tramadol) Etoricoxib Lumiracoxib ‡ Parecoxib Rofecoxib ‡ Valdecoxib ‡ Fenamates Flufenamic acid Meclofenamic acid Mefenamic acid...
122 KB (12,155 words) - 00:35, 29 October 2024
Horgan K, et al. (March 2005). "Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial". The New England Journal...
121 KB (12,011 words) - 19:53, 11 October 2024
cerivastatin (brand names Baycol and Lipobay), troglitazone (Rezulin) and rofecoxib (Vioxx).[citation needed] The entire process of developing a drug from...
32 KB (3,829 words) - 15:51, 28 June 2024
indomethacin, the use of celecoxib 400–800 mg per day (Celebrex) and rofecoxib 50 mg per day (Vioxx - no longer available) have both been shown to be...
13 KB (1,500 words) - 21:49, 27 July 2024
COX-2 selective similar to celecoxib and other "COX-2 inhibitors." Unlike rofecoxib, both etodolac and celecoxib can fully inhibit COX-1 and are designated...
10 KB (870 words) - 22:39, 9 August 2024
COX-2 inhibitors Celecoxib (+tramadol) Etoricoxib Lumiracoxib ‡ Parecoxib Rofecoxib ‡ Valdecoxib ‡ Fenamates Flufenamic acid Meclofenamic acid Mefenamic acid...
95 KB (9,781 words) - 14:36, 30 October 2024
COX-2 inhibitors Celecoxib (+tramadol) Etoricoxib Lumiracoxib ‡ Parecoxib Rofecoxib ‡ Valdecoxib ‡ Fenamates Flufenamic acid Meclofenamic acid Mefenamic acid...
31 KB (2,646 words) - 00:18, 26 October 2024