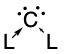In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula...
22 KB (2,401 words) - 05:50, 13 August 2024
persistent carbene (also known as stable carbene) is an organic molecule whose natural resonance structure has a carbon atom with incomplete octet (a carbene),...
62 KB (6,939 words) - 17:20, 16 December 2024
A carbene dye is a reactive dye based on carbene chemistry. A benzophenone is functionalised with a chromophore or group that can be easily converted to...
2 KB (201 words) - 20:10, 2 February 2024
transition metal carbene complex is an organometallic compound featuring a divalent carbon ligand, itself also called a carbene. Carbene complexes have...
20 KB (1,995 words) - 05:52, 13 August 2024
carbene is a type of transition metal carbene complex, which is an organometallic compound containing a divalent organic ligand. In a Fischer carbene...
12 KB (1,438 words) - 05:42, 13 August 2024
Carbene radicals are a special class of organometallic carbenes. The carbene radical can be formed by one-electron reduction of Fischer-type carbenes...
10 KB (983 words) - 22:19, 19 June 2023
Cyclic(alkyl)(amino) carbenes (CAACs) are a class of stable singlet carbene ligands that feature one amino and one sp3 alkyl group adjacent to the carbene carbon atom...
45 KB (4,551 words) - 09:15, 24 December 2024
Coinage metal N-heterocyclic carbene (NHC) complexes refer to transition metal complexes incorporating at least one coinage metal center (M = Cu, Ag,...
24 KB (2,695 words) - 02:20, 25 January 2024
In chemistry, mesoionic carbenes (MICs) are a type of reactive intermediate that are related to N-heterocyclic carbenes (NHCs); thus, MICs are also referred...
16 KB (2,062 words) - 07:46, 10 October 2022
Carbene C−H insertion in organic chemistry concerns the insertion reaction of a carbene into a carbon–hydrogen bond. This organic reaction is of some...
4 KB (501 words) - 13:50, 15 April 2024
Methylene (compound) (category Carbenes)
Methylene (IUPAC name: Methylidene, also called carbene or methene) is an organic compound with the chemical formula CH 2 (also written [CH 2]). It is...
16 KB (1,658 words) - 21:51, 25 November 2024
Methyl phenyldiazoacetate (redirect from Donor-acceptor carbene)
phenyldiazoacetate and many related derivatives are precursors to donor-acceptor carbenes, which can be used for cyclopropanation or to insert into C-H bonds of...
2 KB (216 words) - 02:01, 23 October 2024
Carbene dimerization is a type of organic reaction in which two carbene or carbenoid precursors react in a formal dimerization to an alkene. This reaction...
3 KB (362 words) - 20:09, 2 February 2024
A foiled carbene in organic chemistry is a special type of stabilized carbene due to the proximity of a double bond. This type of reactive intermediate...
2 KB (228 words) - 19:32, 27 October 2022
An N-heterocyclic carbene boryl anion is an isoelectronic structure of an N-heterocyclic carbene (NHC), where the carbene carbon is replaced with a boron...
23 KB (2,530 words) - 01:04, 21 December 2024
Diazirine (section Triplet vs singlet carbene products)
precursors for carbenes by loss of a molecule of dinitrogen. For example, irradiation of diazirines with ultraviolet light leads to carbene insertion into...
25 KB (2,590 words) - 17:42, 1 November 2024
Stibinidene (section Carbene stabilized stibinidene)
their adducts are often robust. Attempted synthesis of stibinidenes, like carbenes, gives cyclic oligomeric forms. 6-, 5-, 4-, and 3-membered rings have been...
31 KB (3,047 words) - 17:04, 17 December 2024
Carbene analogs in chemistry are carbenes with the carbon atom replaced by another chemical element. Just as regular carbenes they appear in chemical...
7 KB (753 words) - 22:12, 27 August 2023
Carbones (section Other carbene structures)
ligand, typically a phosphine (carbodiphosphoranes) or a N-heterocyclic carbene/NHC (carbodicarbenes), that stabilises the central carbon atom through...
28 KB (2,814 words) - 11:17, 26 December 2023
reactions is the addition of a singlet carbene to an alkene to make a cyclopropane (see figure at left). A carbene is a neutral molecule containing a divalent...
14 KB (1,611 words) - 13:49, 6 May 2024
Dicationic complexes of Ge(II) have been isolated with bulky isocyanide and carbene ligands. Much more weakly coordinated Germanium (II) dications have been...
19 KB (2,255 words) - 11:21, 17 December 2023
Cyclopropanation (section Using free carbenes)
exist for converting alkenes to cyclopropane rings using carbene type reagents. As carbenes themselves are highly reactive it is common for them to be...
14 KB (1,504 words) - 19:07, 5 July 2024
reaction is catalyzed by nucleophiles such as a cyanide or an N-heterocyclic carbene (usually thiazolium salts). The reaction mechanism was proposed in 1903...
7 KB (792 words) - 09:50, 22 November 2024
Umpolung (section N-heterocyclic carbenes)
formed between two carbons that are normally electrophiles. N-heterocyclic carbenes are similar to cyanide in reactivity. Like cyanide, they have an unusual...
14 KB (1,784 words) - 16:19, 18 November 2024
Diazo (section As carbene precursors)
carbene dimerization reaction. Diazo compounds are intermediates in the Bamford–Stevens reaction of tosylhydrazones to alkenes, again with a carbene intermediate:...
13 KB (1,439 words) - 01:23, 17 July 2024
The methylene group should be distinguished from the CH2 molecule called carbene. This was also formerly called methylene. The central carbon in 1,3-dicarbonyl...
2 KB (215 words) - 23:50, 10 September 2024
used to access 7-membered rings. The first step involves formation of a carbene from ethyl diazoacetate, which cyclopropanates an aromatic ring. The ring...
16 KB (1,706 words) - 10:31, 13 September 2024
Multiplicity (chemistry) (section Carbenes)
into singlet carbenes and triplet carbenes, named for their spin multiplicities. Both have two non-bonding electrons; in singlet carbenes these exist as...
7 KB (814 words) - 22:54, 5 September 2023
Carbenoid (category Carbenes)
is a reactive intermediate that shares reaction characteristics with a carbene. In the Simmons–Smith reaction the carbenoid intermediate is a zinc / iodine...
2 KB (194 words) - 15:43, 5 May 2023
reaction of the Fischer carbene complexes) is the chemical reaction of an aromatic or vinylic alkoxy pentacarbonyl chromium carbene complex with an alkyne...
13 KB (1,503 words) - 22:31, 27 September 2024