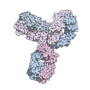
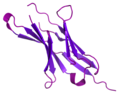
Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody used in cancer immunotherapy that treats melanoma, lung cancer, head and neck...
79 KB (7,328 words) - 01:51, 16 July 2024
system. Atezolizumab is an anti PD-L1 monoclonal antibody. Nivolumab and Pembrolizumab are anti PD-1 monoclonal antibodies. Ipilimumab is a monoclonal antibody...
50 KB (5,300 words) - 12:47, 16 August 2024
As adjuvant therapy, mRNA-4157 monotherapy and in combination with pembrolizumab have been investigated in patients with resected solid tumors (melanoma...
9 KB (899 words) - 20:37, 28 April 2024
treatment options. Pembrolizumab monotherapy is an appealing approach for these patients, with median OS of 30 months with pembrolizumab single agent compared...
59 KB (6,487 words) - 18:42, 27 December 2023
Cancer immunotherapy (section Pembrolizumab)
checkpoint inhibitors include antibodies such as ipilimumab, nivolumab, and pembrolizumab. Dendritic cell therapy provokes anti-tumor responses by causing dendritic...
89 KB (10,338 words) - 14:21, 16 July 2024
immunotherapy drugs blocking LAG3, B7-H3, KIR, OX40, PARP, CD27, and ICOS. Pembrolizumab (Keytruda, formerly MK-3475 and lambrolizumab) was developed by Merck...
31 KB (3,266 words) - 05:29, 20 May 2024
Favezelimab/pembrolizumab is a fixed-dose combination of two monoclonal antibodies developed by Merck to treat various cancers. Timmerman, John; Lavie...
7 KB (422 words) - 20:10, 24 April 2024
"Pilot Study of Pembrolizumab in Untreated Extranodal, NK/T Cell Lymphoma, Nasal Type". 12 May 2021. "Phase I/II Study of Pembrolizumab in Patients with...
46 KB (5,491 words) - 20:47, 14 June 2024
It is being tested by itself and in a fixed-dose combination with pembrolizumab. Garralda, E.; Sukari, A.; Lakhani, N. J.; Patnaik, A.; Lou, Y.; Im...
7 KB (511 words) - 20:09, 24 April 2024
liver, and that he had begun treatment with the immunotherapy drug pembrolizumab and was about to start radiation therapy. His healthcare was managed...
328 KB (28,786 words) - 12:49, 18 August 2024
drugs or products, each with over $1 billion in revenue: Keytruda (pembrolizumab), a humanized antibody used in cancer immunotherapy that had $14.3 billion...
96 KB (9,392 words) - 08:18, 3 August 2024
better using immunotherapy, undergoing treatment every three weeks with pembrolizumab. Two Sides of If (2005) "Take No Prisoners"/"Killing Time" (1981) "Whiskey...
19 KB (2,004 words) - 14:41, 4 August 2024
clinical and observational studies, immune checkpoint inhibitors (e.g., pembrolizumab) have been tested in mucosal melanomas and have shown promising response...
5 KB (435 words) - 00:01, 9 June 2024
for those whose tumors express PD-L1. And the similar immunotherapy pembrolizumab for those whose tumors have mutations in various DNA repair pathways...
131 KB (14,807 words) - 00:05, 15 August 2024
PD-L1, but are sometimes effective in those that do not. Treatment with pembrolizumab, atezolizumab, or combination nivolumab plus ipilimumab are all superior...
90 KB (9,784 words) - 14:55, 21 June 2024
response rate with median progression free survival of 10.5 months Pembrolizumab: 12% objective response rate with median progression free survival of...
21 KB (2,187 words) - 14:39, 28 May 2024
quality-assured biosimilars Afatinib and gefitinib are alternatives Pembrolizumab is an alternative, including quality-assured biosimilars Enzalutamide...
67 KB (4,835 words) - 05:59, 17 August 2024
repair benefit from treatment with the immune checkpoint inhibitor drug pembrolizumab and PARP inhibitors, namely olaparib, rucaparib, or niraparib. Bone...
76 KB (8,857 words) - 08:48, 12 August 2024
often costly. For example, one immune check point inhibitor treatment, pembrolizumab, costs US$10,000 to $12,000 for a single dose administered every 3 weeks...
154 KB (16,076 words) - 04:50, 7 August 2024
advanced NSCLC but with high PD-L1 levels, who will be treated with pembrolizumab in combination with CIMAvax instead of nivolumab. As of 2023, accural...
16 KB (1,556 words) - 23:09, 17 July 2024
for "A Study of mRNA-5671/V941 as Monotherapy and in Combination With Pembrolizumab (V941-001)" at ClinicalTrials.gov https://www.fiercebiotech...
3 KB (223 words) - 08:17, 19 May 2024
2014 and by the US FDA in December 2014 to treat metastatic melanoma. Pembrolizumab (Keytruda, MK-3475, Merck), which also targets PD-1 receptors, was approved...
39 KB (5,010 words) - 05:27, 17 August 2024
cytotoxic component MMAE, and the checkpoint inhibitors, Nivolumab and Pembrolizumab. This has been an important step in the treatment for the few, but still...
87 KB (8,529 words) - 13:45, 12 August 2024
licensed monoclonal antibodies include: Pembrolizumab (Keytruda) binds to PD-1 proteins found on T cells. Pembrolizumab blocks PD-1 and help the immune system...
20 KB (2,234 words) - 01:21, 22 July 2024
Pemetrexed is also recommended in combination with carboplatin and pembrolizumab for the first-line treatment of advanced non-small cell lung cancer...
17 KB (1,250 words) - 18:48, 25 May 2024
approved for medical use include Pembrolizumab, Larotrectinib, Selpercatinib, Entrectinib, and Pralsetinib. Pembrolizumab was approved by the US Food and...
7 KB (597 words) - 15:52, 6 August 2024
cancer with mismatch repair deficiency and microsatellite instability. Pembrolizumab is approved for advanced CRC tumours that are MMR deficient and have...
145 KB (15,793 words) - 06:45, 16 August 2024
(e.g. sunitinib), checkpoint inhibitors (e.g. ipilimumab, nivolumab, pembrolizumab, atezolizumab) Contrast agent Gadolininum based contrast agent Other...
20 KB (2,303 words) - 18:34, 27 July 2024
metastatic melanoma include the biologic immunotherapy agents ipilimumab, pembrolizumab, and nivolumab; BRAF inhibitors, such as vemurafenib and dabrafenib;...
15 KB (1,427 words) - 00:09, 18 August 2024
pembrolizumab may have only minimal effects on the rate of death resulting from treatment or the rate at which the cancer advances. Pembrolizumab may...
132 KB (13,036 words) - 08:44, 12 August 2024