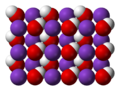
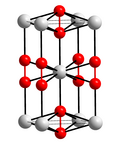
Caesium hydroxide is a strong base (pKa= 15.76) containing the highly reactive alkali metal caesium, much like the other alkali metal hydroxides such as...
6 KB (386 words) - 19:57, 15 January 2025
temperature of caesium is −116 °C (−177 °F), and it ignites explosively in air to form caesium hydroxide and various oxides. Caesium hydroxide is a very strong...
93 KB (10,025 words) - 17:29, 6 December 2024
Potassium hydroxide is an inorganic compound with the formula KOH, and is commonly called caustic potash. Along with sodium hydroxide (NaOH), KOH is a...
21 KB (2,063 words) - 06:28, 7 October 2024
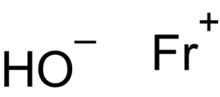
Lithium hydroxide (LiOH) Sodium hydroxide (NaOH) Potassium hydroxide (KOH) Rubidium hydroxide (RbOH) Caesium hydroxide (CsOH) Francium hydroxide (FrOH)...
3 KB (363 words) - 13:40, 16 July 2024
application. Caesium-137 reacts with water, producing a water-soluble compound (caesium hydroxide). The biological behaviour of caesium is similar to...
36 KB (3,877 words) - 13:26, 7 January 2025
Caesium chloride or cesium chloride is the inorganic compound with the formula CsCl. This colorless salt is an important source of caesium ions in a variety...
39 KB (3,421 words) - 15:09, 16 December 2024
Lithium hydroxide is an inorganic compound with the formula LiOH. It can exist as anhydrous or hydrated, and both forms are white hygroscopic solids....
14 KB (1,089 words) - 12:21, 25 August 2024
electronegativity. Caesium fluoride can be prepared by the reaction of caesium hydroxide (CsOH) with hydrofluoric acid (HF) and the resulting salt can then...
10 KB (898 words) - 23:26, 19 December 2024
francium has never been made. Francium hydroxide's alkalinity is predicted to be stronger than caesium hydroxide. Robert Krebs (2006), "Francium", The...
2 KB (152 words) - 21:11, 24 December 2024
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of...
54 KB (6,013 words) - 16:09, 19 January 2025
It can also be synthesized by reacting caesium hydroxide with carbon dioxide. 2 CsOH + CO2 → Cs2CO3 + H2O Caesium carbonate facilitates the N-alkylation...
12 KB (1,075 words) - 10:03, 9 January 2025
peroxide and caesium hydroxide. 2 CsO2 + 2 H2O → O2↑ + H2O2 + 2 CsOH Heating to approximately 400 °C induces thermal decomposition to caesium peroxide. The...
3 KB (195 words) - 11:21, 29 December 2023
CH3COOCs. It is a white solid that may be formed by the reaction of caesium hydroxide or caesium carbonate with acetic acid. It is used in organic synthesis....
5 KB (284 words) - 08:46, 7 September 2024
Caesium monoxide or caesium oxide is a chemical compound with the chemical formula Cs2O. It is the simplest and most common oxide of the caesium. It forms...
5 KB (275 words) - 14:41, 14 November 2024
Alkali metal (section Hydroxides)
Because metallic caesium is too reactive to handle, it is normally offered as caesium azide (CsN3). Caesium hydroxide is formed when caesium interacts aggressively...
214 KB (23,532 words) - 22:45, 29 January 2025
bases Lithium hydroxide, LiOH Sodium hydroxide, NaOH Potassium hydroxide, KOH Rubidium hydroxide, RbOH Caesium hydroxide, CsOH Calcium hydroxide, Ca(OH)2 Strontium...
3 KB (369 words) - 22:16, 23 May 2024
produce potassium hydroxide: K2O + H2O → 2 KOH Rubidium oxide reacts with water to produce rubidium hydroxide: Rb2O + H2O → 2 RbOH Caesium oxide reacts with...
5 KB (573 words) - 11:18, 30 August 2024
lattice and the attractions between water molecules. Dissolving potassium hydroxide is exothermic, as more energy is released during solvation than is used...
5 KB (681 words) - 12:45, 26 March 2024
reacting ozone with caesium superoxide: CsO2 + O3 → CsO3 + O2 The compound reacts strongly with any water in the air forming caesium hydroxide. 4 CsO3 + 2 H2O...
4 KB (314 words) - 11:34, 8 December 2023
reaction between caesium chloride (CsCl) and silver tungstate (Ag2WO4) or the reaction between tungstic acid and caesium hydroxide. Cesium tungstate v t e...
2 KB (90 words) - 06:44, 24 November 2024
semiconductor with band gap 2.6 eV. The compound hydrolyzes readily, yielding caesium hydroxide, metallic gold, and hydrogen. 2 CsAu + 2 H2O → 2 CsOH + 2 Au + H2...
4 KB (301 words) - 05:26, 24 November 2024
for the hydroxide ion (Epa = 1635 kJ/mol), one of the strongest known proton acceptors in the gas phase. Suspensions of potassium hydroxide in dimethyl...
15 KB (1,028 words) - 15:15, 24 April 2024
Mercury(II) hydroxide or mercuric hydroxide is the metal hydroxide with the chemical formula Hg(OH)2. The compound has not been isolated in pure form,...
2 KB (173 words) - 20:58, 1 January 2022
neutralization reaction of selenic acid and caesium hydroxide: 2 CsOH + H2SeO4 → Cs2SeO4 + 2 H2O caesium selenate can precipitate compounds such as CsLiSeO4·1⁄2H2O...
3 KB (282 words) - 06:20, 24 November 2024
structure. Caesium azide can be prepared from the neutralization reaction between hydrazoic acid and caesium hydroxide: CsOH + HN3 → CsN3 + H2O Caesium carbonate...
3 KB (276 words) - 20:50, 12 May 2024
Francium (redirect from Eka-caesium)
22 minutes. It is the second-most electropositive element, behind only caesium, and is the second rarest naturally occurring element (after astatine)...
38 KB (4,272 words) - 09:46, 29 January 2025
spellings may differ, such as aluminum/aluminium, sulfur/sulphur, and caesium/cesium. Contents A B C Ca–Cu D E F G H I K L M N O P R S T U V W X Y Z...
183 KB (107 words) - 15:38, 19 January 2025
CsNO2 caesium nitrite 13454–83–6 CsNO3 caesium nitrate 7789–18–6 CsN3 caesium azide 22750–57–8 CsOH caesium hydroxide 21351–79–1 CsO2 caesium superoxide...
139 KB (120 words) - 17:07, 15 July 2024
with lithium diphenylphosphide, or diphenylphosphine in presence of caesium hydroxide. It reacts with copper(I) iodide to give a luminescent dinuclear complex...
4 KB (258 words) - 07:18, 11 October 2022
sugar. Caesium cyanide has chemical properties similar to potassium cyanide and is very toxic. Hydrogen cyanide reacts with cesium hydroxide giving cesium...
2 KB (100 words) - 19:17, 12 May 2024