Daclatasvir, sold under the brand name Daklinza, is an antiviral medication used in combination with other medications to treat hepatitis C (HCV). The...
25 KB (2,673 words) - 05:26, 10 April 2024
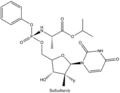
Daclatasvir/sofosbuvir (trade names Darvoni, Sovodak) is a two-drug combination for the treatment of hepatitis C. It is given as a single daily pill containing...
8 KB (741 words) - 14:59, 24 January 2024
products include velpatasvir/sofosbuvir, sofosbuvir/daclatasvir, osimertinib, crizotinib, daclatasvir, sofosbuvir, afatinib, axitinib, brigatinib, baricitinib...
5 KB (232 words) - 10:23, 8 March 2024
Valganciclovirα Entecavir Tenofovir disoproxil fumarate Daclatasvir Daclatasvir/sofosbuvir (daclatasvir + sofosbuvir) Glecaprevir/pibrentasvir (glecaprevir...
67 KB (4,835 words) - 06:05, 31 October 2024
those who have been previously infected. In combination with ledipasvir, daclatasvir or simeprevir, it is not recommended with amiodarone due to the risk...
64 KB (5,828 words) - 23:44, 21 October 2024
ledipasvir/sofosbuvir, or sofosbuvir/velpatasvir. Sofosbuvir with either daclatasvir or simeprevir may also be used. HCV genotype 1a (with compensated cirrhosis):...
109 KB (11,845 words) - 00:33, 19 October 2024
post-marketing trial of beclabuvir, in combination with asunaprevir and daclatasvir, to study the combination's safety profile with regard to liver function...
4 KB (311 words) - 20:41, 1 December 2023
interferon-free regimens with other direct-acting antiviral agents including daclatasvir. "Prescription medicines: registration of new chemical entities in Australia...
7 KB (271 words) - 11:27, 21 October 2024
act on dimers of NS5A. A number of modeling studies have shown that daclatasvir, which is an NS5A inhibitor, only binds to the "back-to-back" NS5A dimer...
50 KB (5,210 words) - 15:08, 16 May 2024
Lamivudine: cytidine analogue RTI Zidovudine: thymidine analogue RTI Daclatasvir (Daklinza) Hepatitis C NS5A inhibitor Darunavir HIV antiretroviral HIV...
13 KB (513 words) - 14:31, 4 May 2024
trimethoprim) Ribavirin Oseltamivirα Valganciclovirα Entecavir Daclatasvir Daclatasvir/sofosbuvir (daclatasvir + sofosbuvir) Glecaprevir/pibrentasvir (glecaprevir...
38 KB (2,574 words) - 03:03, 15 July 2024
hepatitis C drugs ledipasvir/sofosbuvir or sofosbuvir along with amiodarone, daclatasvir, or simeprevir developed abnormally slow heartbeats and one died of cardiac...
26 KB (2,541 words) - 05:04, 4 January 2024
Boceprevir J05AP04 Faldaprevir J05AP05 Simeprevir J05AP06 Asunaprevir J05AP07 Daclatasvir J05AP08 Sofosbuvir J05AP09 Dasabuvir J05AP10 Elbasvir J05AP11 Grazoprevir...
6 KB (538 words) - 04:59, 18 December 2023
individual patients obtain legal access to generic versions of sofosbuvir, daclatasvir, and ledipasvir. At EASL International Liver Congress, Dr. Freeman presented...
11 KB (1,151 words) - 03:58, 24 September 2024
boceprevir, simeprevir, and others NS5A inhibitors, including ledipasvir, daclatasvir, and others NS5B inhibitors, including sofosbuvir, dasabuvir, and others...
165 KB (16,882 words) - 20:39, 31 October 2024
Nguyen Tien (May 2018). "Beclabuvir in combination with asunaprevir and daclatasvir for hepatitis C virus genotype 1 infection: A systematic review and meta-analysis"...
54 KB (6,612 words) - 08:16, 2 November 2024
determination of ledipasvir/sofosbuvir in human plasma using antiviral daclatasvir as an internal standard. Average extraction recoveries for sofosbuvir...
22 KB (1,936 words) - 13:18, 6 January 2024
Gilead Sciences Azactam (aztreonam) Baraclude (entecavir) Daklinza (daclatasvir) Evotaz (atazanavir/cobicistat) Megace (megestrol acetate) Reyataz (atazanavir)...
97 KB (7,693 words) - 20:50, 25 October 2024
Index Unitaid Examples of drug controversies: Sofosbuvir § Economics Daclatasvir § Economics Epinephrine autoinjector § Price Insulin (medication) § Economics:...
52 KB (5,899 words) - 01:24, 21 October 2024
remissions in <50% of cases. More recently, drugs (e.g. simeprevir, daclatasvir, sofosbuvir, and dasabuvir) that directly inhibit the virus's reproduction...
116 KB (14,600 words) - 02:13, 16 October 2024
in a FDC with paritaprevir and ritonavir, co-packaged with dasabuvir Daclatasvir, approved on July 24, 2015 Elbasvir, approved on January 28, 2016 in...
10 KB (1,179 words) - 06:28, 28 March 2024
Coronaviruses species possess an intrinsic resistance to ribavirin. Sofosbuvir/daclatasvir is a drug combination developed to treat hepatitis C. In October 2020...
128 KB (14,818 words) - 14:23, 18 October 2024
all with last generation of antiviral drugs (sofosbuvir, ledipasvir, daclatasvir, etc.). While some countries established a moratorium delaying treatments...
18 KB (2,192 words) - 15:15, 26 May 2024
January 2016). "Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C." Journal of Virus Eradication. 2 (1): 28–31. doi:10...
11 KB (1,080 words) - 12:29, 15 June 2024
inhibitor ledipasvir was approved. Four other NS5A inhibitors of this class—daclatasvir, ombitasvir, elbasvir and velpatasvir—have been subsequently approved...
33 KB (3,798 words) - 21:57, 16 May 2024
including generic treatment including active substances - sofosbuvir, daclatasvir, ledipasvir, velpatasvir. The results of using antiviral therapy reach...
22 KB (2,250 words) - 13:08, 4 November 2024
NS5A Y93H variant are less likely to respond to NS5A inhibitors such as daclatasvir, ledipasvir and ombitasvir, which are commonly used in popular DAA regimens...
53 KB (6,064 words) - 16:19, 13 August 2024
antibodies, Viremic persons received sofosbuvir (400 mg daily) plus daclatasvir (60 mg daily) with or without ribavirin for 12 or 24 weeks, Almost 50...
16 KB (1,664 words) - 07:22, 10 July 2024
succeeded in the offering of variable DAAs (as sofosbuvir, simeprevir, daclatasvir, ombitasvir and paritaprevir, ledipasvir) in Egypt at affordable prices...
8 KB (936 words) - 04:02, 6 March 2023
PMID 28276427. Monga J, Valeriote F, Hwang C, Gadgeel S, Ghosh J (2023). "Daclatasvir, an Antiviral Drug, Downregulates Tribbles 2 Pseudokinase and Resensitizes...
8 KB (923 words) - 07:20, 3 June 2024