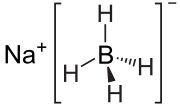
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula NaBH4 (sometimes written...
27 KB (2,615 words) - 13:44, 24 January 2024
Borohydride refers to the anion [BH4]−, which is also called tetrahydridoborate, and its salts. Borohydride or hydroborate is also the term used for compounds...
8 KB (812 words) - 10:07, 30 January 2024
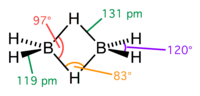
Unlike most other metal–borohydrides, which are ionic structures, aluminium borohydride is a covalent compound. Aluminium borohydride is formed by the reaction...
5 KB (301 words) - 09:38, 14 April 2023
Lithium borohydride (LiBH4) is a borohydride and known in organic synthesis as a reducing agent for esters. Although less common than the related sodium...
10 KB (827 words) - 02:07, 31 October 2023
Beryllium borohydride is an inorganic compound with the chemical formula Be[BH4]2. Beryllium borohydride is formed by the reaction of beryllium hydride...
3 KB (202 words) - 21:46, 4 April 2024
Uranium borohydride is the inorganic compound with the empirical formula U(BH4)4. Two polymeric forms are known, as well as a monomeric derivative that...
5 KB (476 words) - 01:09, 3 March 2024
nonreactive with certain molten salts such as lithium fluoride, lithium borohydride, and sodium hydride. With a molar mass of 7.95 g/mol, it is the lightest...
19 KB (1,932 words) - 20:03, 23 July 2024
Alternatively, a small amount of diborane product can be added to form lithium borohydride, which will decompose the fluoroborate and make the reaction autocatalytic...
27 KB (2,596 words) - 17:47, 18 June 2024
Direct borohydride fuel cells (DBFCs) are a subcategory of alkaline fuel cells which are directly fed by sodium borohydride or potassium borohydride as a...
5 KB (672 words) - 14:28, 25 May 2024
Sodium triacetoxyborohydride (category Borohydrides)
other borohydrides, it is used as a reducing agent in organic synthesis. This colourless salt is prepared by protonolysis of sodium borohydride with acetic...
5 KB (385 words) - 14:51, 24 January 2024
in vacuum. Although not performed industrially, hydrolysis of sodium borohydride Na[BH4] with a suitable catalyst gives sodium metaborate and hydrogen...
13 KB (1,436 words) - 01:33, 15 August 2023
informed that there was no longer a need for uranium borohydride, but it appeared that sodium borohydride could be useful in generating hydrogen. They began...
15 KB (1,386 words) - 05:55, 9 June 2024
Reductive amination (section Sodium Borohydride)
be isolated and reduced with a suitable reducing agent (e.g., sodium borohydride) to produce the final amine product. Intramolecular reductive amination...
21 KB (2,134 words) - 00:28, 26 May 2024
Sodium cyanoborohydride (category Borohydrides)
For example, Na[BH3(CN)] is less reducing than its counterpart sodium borohydride, containing [BH4]−. Sodium cyanoborohydride is a mild reducing agent...
9 KB (792 words) - 14:48, 1 April 2024
Reitz AB (April 2004). "Reductive aminations of carbonyl compounds with borohydride and borane reducing agents". Organic Reactions. 59. Hoboken, New Jersey...
166 KB (15,881 words) - 05:25, 26 July 2024
be produced in the laboratory by reducing cyanocobalamin with sodium borohydride in alkaline solution, followed by the addition of methyl iodide. This...
10 KB (836 words) - 22:47, 23 January 2024
wool, either as gas or from solutions of sodium dithionite, and sodium borohydride. Bleaches generally react with many other organic substances besides...
36 KB (3,886 words) - 15:18, 3 July 2024
by controlled potential reduction, or chemical reduction using sodium borohydride in alkaline solution, zinc in acetic acid, or by the action of thiols...
30 KB (2,638 words) - 05:07, 27 May 2024
process (see direct borohydride fuel cell) creates hydrogen as needed, but has other issues, such as the high price of the sodium borohydride that is the raw...
99 KB (12,922 words) - 15:51, 26 July 2024
in THF. Alternatively, it can be prepared by the oxidation of sodium borohydride with iodine in THF. The complex can reduce carboxylic acids to alcohols...
4 KB (328 words) - 15:55, 13 May 2023
camphorquinone . Camphor can also be reduced to isoborneol using sodium borohydride. In biosynthesis, camphor is produced from geranyl pyrophosphate, via...
32 KB (3,027 words) - 11:45, 20 July 2024
which has been demonstrated through the application of either sodium borohydride or vitamin C. There are variations both in degree of cooking and in the...
20 KB (2,121 words) - 16:51, 24 July 2024
reagents. Prominent among these reagents are the alkali metal salts of borohydrides and aluminium hydrides. In terms of reaction mechanism, metal hydrides...
24 KB (2,715 words) - 12:53, 24 June 2024
with a green flame. It is an intermediate in the preparation of sodium borohydride and is a popular reagent in organic chemistry. It is a weak Lewis acid...
4 KB (355 words) - 11:33, 12 June 2024
chemistry. Metallic sodium is used mainly for the production of sodium borohydride, sodium azide, indigo, and triphenylphosphine. A once-common use was...
70 KB (8,195 words) - 02:53, 15 July 2024
require sodium borohydride as a stabilizer, which could result in undesired side reactions. In contrast, BH3·THF requires sodium borohydride to inhibit reduction...
7 KB (591 words) - 22:09, 24 April 2024
catalysts are typically prepared by reacting a salt of nickel with sodium borohydride. The composition and properties vary depending on the specific preparation...
13 KB (1,533 words) - 19:12, 13 September 2023
Nozaki-Hiyama reaction. Aldehydes or ketones are reduced with sodium borohydride or lithium aluminium hydride (after an acidic workup). Another reduction...
34 KB (3,818 words) - 21:33, 7 July 2024
mild hydride-reducing agents, such as sodium borohydride, potassium borohydride, and lithium borohydride to dihydroartemisinin (a lactol) in over 90%...
10 KB (998 words) - 09:49, 1 July 2024
behave as hydrogen-atom donors and act as acids. Hydrides such as sodium borohydride, lithium aluminium hydride, diisobutylaluminium hydride (DIBAL) and super...
21 KB (2,333 words) - 12:44, 22 May 2024