Butyllithium may refer to one of 5 isomeric organolithium reagents of which 3 are commonly used in chemical synthesis: n-Butyllithium, abbreviated BuLi...
495 bytes (83 words) - 11:36, 2 December 2016
n-Butyllithium C4H9Li (abbreviated n-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers...
18 KB (1,856 words) - 02:21, 23 October 2024
tert-Butyllithium is a chemical compound with the formula (CH3)3CLi. As an organolithium compound, it has applications in organic synthesis since it is...
11 KB (1,038 words) - 18:59, 3 December 2024
sec-Butyllithium is an organometallic compound with the formula CH3CHLiCH2CH3, abbreviated sec-BuLi or s-BuLi. This chiral organolithium reagent is used...
8 KB (642 words) - 16:37, 19 December 2023
hydrides (diethylaluminium hydride, trimethylaluminium, triethylaluminium, butyllithium), with a few exceptions (i.e. dimethylmercury and tetraethyllead) Copper...
9 KB (814 words) - 16:19, 28 November 2024
reagents are the butyllithiums. tert-Butyllithium and sec-butyllithium are generally more reactive and have better selectivity than n-butyllithium, however,...
55 KB (5,971 words) - 20:35, 22 July 2024
commonly used. Lithium amide, LiHMDS, or organolithium reagents, such as butyllithium (BuLi), are frequently used to form lithium acetylides: HC≡CH + BuLi...
12 KB (1,306 words) - 03:41, 4 December 2024
formula C4H9Li (molar mass: 64.06 g/mol) may refer to: n-Butyllithium sec-Butyllithium tert-Butyllithium This set index page lists chemical structure articles...
347 bytes (54 words) - 14:41, 6 July 2019
aluminium hydride (LiAlH4), lithium triethylborohydride, n-butyllithium and tert-butyllithium. Metallic lithium and its complex hydrides, such as lithium...
141 KB (13,737 words) - 00:44, 14 December 2024
bidentate ligand. TMEDA has an affinity for lithium ions. When mixed with n-butyllithium, TMEDA's nitrogen atoms coordinate to the lithium, forming a cluster...
8 KB (589 words) - 23:59, 29 September 2024
of organometallic compounds include organolithium compounds such as n-butyllithium (n-BuLi), organozinc compounds such as diethylzinc (Et2Zn), organotin...
31 KB (3,157 words) - 20:03, 24 September 2024
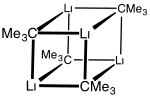
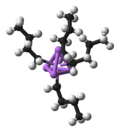
Organolithium reagents are most often found in polymeric form, such as n-butyllithium shown here...
29 KB (3,220 words) - 00:35, 17 November 2024
synthesised by the deprotonation of 2,2,6,6-tetramethylpiperidine with n-butyllithium at −78 °C. Recent reports show that this reaction can also be performed...
3 KB (161 words) - 17:01, 7 October 2022
[citation needed] Anionic chain polymerization, which is initiated by n-Butyllithium, produces cis-1,4-polyisoprene dominant polyisoprene. 90–92% of repeating...
5 KB (502 words) - 19:27, 10 July 2024
condensation reaction of amines and formaldehyde. It undergoes deprotonation by butyllithium to give a reagent that serves as a source of the formyl anion. V. Subramanian...
1 KB (84 words) - 09:17, 2 September 2024
compounds are extremely powerful bases and nucleophiles. For use as bases, butyllithiums are often used and are commercially available. An example of an organolithium...
214 KB (23,524 words) - 08:55, 14 December 2024
reaction of preformed organolithium compounds: R−Li + R′−X → R−X + R′−Li Butyllithium is commonly used. Gilman and Wittig independently discovered this method...
10 KB (1,200 words) - 17:34, 25 September 2023
(scheme 1), the alkyne proton of ethyl propiolate is deprotonated by n-butyllithium at -78 °C to form lithium ethyl propiolate to which cyclopentanone is...
12 KB (1,249 words) - 07:49, 9 May 2024
gamma-Hydroxybutyric acid 591-81-1 C4H9ClHg n-Butylmercuric chloride 543-63-5 C4H9Li n-butyllithium C4H9NO2 γ-aminobutyric acid 56-12-2 C4H9NO3 threonine Thr 72-19-5 C4H9Na...
183 KB (107 words) - 07:29, 24 November 2024
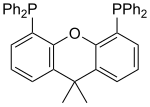
prepared by double directed lithiation of 9,9-dimethylxanthene with sec-butyllithium followed by treatment with chlorodiphenylphosphine. Birkholz, Mandy-Nicole;...
3 KB (253 words) - 19:26, 24 April 2023
anionic copolymerization. Typically, an organometallic compound such as butyllithium is used as a catalyst. Butadiene is then added and after styrene again...
82 KB (8,579 words) - 01:22, 11 November 2024
the strongest Arrhenius base; however, a number of compounds such as n-butyllithium, sodium amide, sodium hydride, caesium hydride, etc., which cannot be...
93 KB (10,025 words) - 17:29, 6 December 2024
participate in reactions with certain strong nucleophiles however. Tert-butyllithium deprotonates DCM: H2CCl2 + RLi → HCCl2Li + RH Methyllithium reacts with...
29 KB (2,592 words) - 02:01, 17 December 2024
very slowly in the presence of moisture. Organometallic reagents like butyllithium (hexameric cluster, [BuLi]6) or methylmagnesium bromide (ether complex...
32 KB (3,469 words) - 22:24, 16 October 2024
Commonly, the mixture called Schlosser's base is produced by combining n-butyllithium and potassium tert-butoxide in a one-to-one ratio. The high reactivity...
3 KB (372 words) - 09:06, 12 April 2020
treatment with hydrogen chloride. It reacts with lithium metal to give n-butyllithium: 2 Li + CH3(CH2)3Cl → CH3(CH2)3Li + LiCl Record in the GESTIS Substance...
4 KB (146 words) - 08:43, 12 August 2023
Lithium, east of the A41 at Wirral International Business Park, makes butyllithium and other organometallic compounds. At Port Sunlight, Unilever make and...
179 KB (17,042 words) - 20:30, 30 November 2024
deprotonated with a strong base such as n-butyllithium: [Ph3P+CH2R]X− + C4H9Li → Ph3P=CHR + LiX + C4H10 Besides n-butyllithium (nBuLi), other strong bases like...
7 KB (714 words) - 02:26, 19 June 2024
now part of Chevron Corporation North British Locomotive Company n-Butyllithium, an organic compound Neutral Buoyancy Laboratory, an astronaut training...
1 KB (164 words) - 14:28, 7 February 2023
three-carbon building block for organic synthesis. Deprotonation with n-butyllithium gives propynyllithium. This nucleophilic reagent adds to carbonyl groups...
9 KB (737 words) - 00:26, 24 August 2024