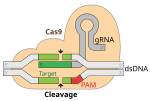
Exagamglogene autotemcel, sold under the brand name Casgevy, is a gene therapy used for the treatment of sickle cell disease and transfusion-dependent...
20 KB (1,447 words) - 04:01, 7 September 2024
cell anemia by inhibiting the BCL11A gene have been developed. Exagamglogene autotemcel, sold under the brand name Casgevy, is a gene therapy for the treatment...
57 KB (5,458 words) - 22:49, 19 September 2024
the Year Award (FOYA) award in 2022. The company’s lead program, exagamglogene autotemcel, or exa-cel (formerly CTX001), was granted regulatory approval...
11 KB (918 words) - 14:13, 19 April 2024
developing red blood cells, with resultant resolution of the anemia. Exagamglogene autotemcel, sold under the brand name Casgevy, is a gene therapy for the treatment...
67 KB (7,132 words) - 02:15, 19 October 2024
like deferoxamine, deferiprone and luspatercept. Gene therapy, exagamglogene autotemcel is approved for medical use in the United Kingdom since November...
32 KB (3,700 words) - 16:00, 26 September 2024
Crizanlizumab B06AX02 Betibeglogene autotemcel B06AX03 Voxelotor B06AX04 Mitapivat B06AX05 Exagamglogene autotemcel "ATC (Anatomical Therapeutic Chemical...
960 bytes (213 words) - 02:49, 18 December 2023
everolimus (USAN) Evex Evista Evolocumab (INN) Evoxac Evrysdi exagamglogene autotemcel (INN) exalamide (INN) exametazime (INN) examorelin (INN) exaprolol...
2 KB (202 words) - 20:44, 27 August 2024
developing red blood cells, with resultant resolution of the anemia. Exagamglogene autotemcel, sold under the brand name Casgevy, is a gene therapy for the treatment...
44 KB (4,881 words) - 19:24, 3 October 2024
dezaparvovec (Hemgenix): AAV-based treatment for hemophilia B Exagamglogene autotemcel (Casgevy): treatment for sickle cell disease. Gendicine: treatment...
10 KB (792 words) - 10:27, 12 December 2023
Exo-cell may be a misspelling of: Exa-cell, exagamglogene autotemcel Exocell, an organism in the video game Cold Fear This disambiguation page lists articles...
159 bytes (52 words) - 10:21, 1 April 2024
injection of the CRISPR-Cas System was confirmed in March 2020. Exagamglogene autotemcel, a CRISPR-based human gene editing therapy, was used for sickle...
176 KB (18,162 words) - 17:07, 18 October 2024
"Vertex and CRISPR Therapeutics Announce US FDA Approval of Casgevy (exagamglogene autotemcel) for the Treatment of Sickle Cell Disease" (Press release). Vertex...
13 KB (1,312 words) - 01:58, 19 October 2024
Therapeutics that led to the discovery and development of Casgevy, (exagamglogene autotemcel; formerly known as CTX001), the first gene editing treatment to...
30 KB (2,519 words) - 23:23, 5 October 2024
to determine their effectiveness. In 2023, both exagamglogene autotemcel and lovotibeglogene autotemcel were approved for the treatment of sickle cell...
142 KB (15,313 words) - 15:07, 11 October 2024
to treat sickle-cell anemia and beta thalassemia. Casgevy, or exagamglogene autotemcel, directly acts on the genes of the stem cells inside the patient's...
170 KB (19,897 words) - 15:07, 10 October 2024