An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept...
24 KB (3,852 words) - 13:29, 20 September 2024
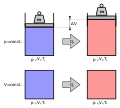
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior...
33 KB (4,571 words) - 21:18, 13 November 2024
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol R or R. It is the molar...
17 KB (1,958 words) - 06:56, 21 October 2024
ideal points Ideal chain, in science, the simplest model describing a polymer Ideal gas law, in physics, governing the pressure of an ideal gas Ideal...
3 KB (435 words) - 02:29, 17 April 2023
possible because he was studying gases in relatively low pressure situations where they behaved in an "ideal" manner. These ideal relationships apply to safety...
52 KB (6,605 words) - 18:21, 5 November 2024
of several empirical gas laws led to the development of the ideal gas law. The ideal gas law was later found to be consistent with atomic and kinetic...
12 KB (1,770 words) - 08:14, 18 November 2024
Partial pressure (redirect from Gas pressure)
pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture (Dalton's Law). The partial pressure of a gas is a measure...
21 KB (2,402 words) - 11:32, 11 October 2024
perfect gas models are ideal gas models in the sense that they all follow the ideal gas equation of state. However, the idea of a perfect gas model is...
8 KB (1,190 words) - 23:54, 7 May 2023
The scale-free ideal gas (SFIG) is a physical model assuming a collection of non-interacting elements with a stochastic proportional growth. It is the...
3 KB (409 words) - 15:12, 9 October 2024
An ideal Bose gas is a quantum-mechanical phase of matter, analogous to a classical ideal gas. It is composed of bosons, which have an integer value of...
22 KB (3,013 words) - 17:08, 6 July 2024
An ideal solution or ideal mixture is a solution that exhibits thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of...
11 KB (2,163 words) - 23:43, 24 September 2024
Avogadro's law (category Gas laws)
experimental gas law relating the volume of a gas to the amount of substance of gas present. The law is a specific case of the ideal gas law. A modern...
10 KB (1,391 words) - 14:05, 14 October 2024
Real gases are nonideal gases whose molecules occupy space and have interactions; consequently, they do not adhere to the ideal gas law. To understand...
15 KB (2,606 words) - 21:06, 4 October 2024
Sackur–Tetrode equation (category Ideal gas)
Sackur–Tetrode equation is an expression for the entropy of a monatomic ideal gas. It is named for Hugo Martin Tetrode (1895–1931) and Otto Sackur (1880–1914)...
9 KB (1,125 words) - 13:50, 18 November 2024
Isothermal process (section Details for an ideal gas)
Δ T = 0 {\displaystyle \Delta T=0} d T = 0 {\displaystyle dT=0} For ideal gases only, internal energy Δ U = 0 {\displaystyle \Delta U=0} while in adiabatic...
16 KB (2,284 words) - 21:53, 13 August 2024
Compressibility factor (redirect from Compressibility factor (gases))
gas deviation factor, describes the deviation of a real gas from ideal gas behaviour. It is simply defined as the ratio of the molar volume of a gas to...
23 KB (2,808 words) - 12:52, 12 September 2024
Boyle's law (category Gas laws)
exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a...
18 KB (2,300 words) - 04:20, 24 October 2024
Degenerate matter (redirect from Degenerate gas)
also applies to metals in the Fermi gas approximation. Degenerate matter is usually modelled as an ideal Fermi gas, an ensemble of non-interacting fermions...
24 KB (3,165 words) - 20:32, 27 October 2024
molar heat capacity of the gas; c V {\displaystyle c_{V}} is constant for an ideal gas. The internal energy of any gas (ideal or not) may be written as...
33 KB (5,007 words) - 16:05, 2 October 2024
states in the blood Inspired and alveolar gases obey the ideal gas law Carbon dioxide (CO2) in the alveolar gas is in equilibrium with the arterial blood...
7 KB (1,016 words) - 18:00, 23 August 2024
Heat capacity ratio (section Ideal-gas relations)
expansion factor and is denoted by γ (gamma) for an ideal gas or κ (kappa), the isentropic exponent for a real gas. The symbol γ is used by aerospace and chemical...
18 KB (2,318 words) - 16:04, 11 August 2024
Non ideal compressible fluid dynamics (NICFD), or non ideal gas dynamics, is a branch of fluid mechanics studying the dynamic behavior of fluids not obeying...
34 KB (3,970 words) - 15:00, 6 August 2024
Molar volume (redirect from Molar gas volume)
_{i}^{0}}=M_{i}v_{i}} For ideal gases, the molar volume is given by the ideal gas equation; this is a good approximation for many common gases at standard temperature...
6 KB (855 words) - 17:00, 9 August 2024
The term ideal machine refers to a hypothetical mechanical system in which energy and power are not lost or dissipated through friction, deformation,...
3 KB (403 words) - 07:15, 12 December 2021
Amagat's law (category Gas laws)
describes the behaviour and properties of mixtures of ideal (as well as some cases of non-ideal) gases. It is of use in chemistry and thermodynamics. It is...
4 KB (728 words) - 15:04, 13 September 2024
describe the classical ideal gas as well as the various quantum ideal gases such as the ideal massive Fermi gas, the ideal massive Bose gas as well as black...
15 KB (2,746 words) - 18:27, 26 April 2024
neutrons in a neutron star, and electrons in a white dwarf. An ideal Fermi gas or free Fermi gas is a physical model assuming a collection of non-interacting...
33 KB (5,618 words) - 12:04, 12 November 2024
Plasma (physics) (redirect from Ideal plasma)
following table summarizes some principal differences: Three factors define an ideal plasma: The plasma approximation: The plasma approximation applies when...
62 KB (6,395 words) - 16:48, 7 November 2024
Joule–Thomson effect (category Gases)
effect) describes the temperature change of a real gas or liquid (as differentiated from an ideal gas) when it is expanding; typically caused by the pressure...
33 KB (4,464 words) - 11:28, 15 August 2024