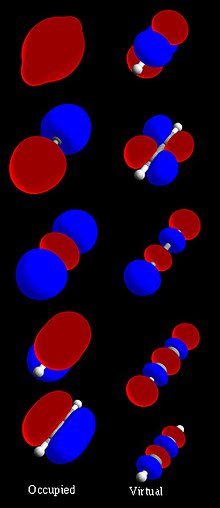
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies,...
33 KB (3,164 words) - 10:14, 24 April 2024
Look up orbital in Wiktionary, the free dictionary. Orbital may refer to: Atomic orbital Molecular orbital Hybrid orbital Orbit Earth orbit Orbit (anatomy)...
1 KB (178 words) - 11:34, 25 November 2023
region. The terms atomic orbital and molecular orbital were introduced by Robert S. Mulliken in 1932 to mean one-electron orbital wave functions. At an elementary...
35 KB (4,390 words) - 11:31, 6 June 2024
000 lbf) HP/PE engine. The company is planning to use hybrids for both sounding and orbital rockets. Orbital Technologies Corporation (Orbitec) has been involved...
56 KB (6,894 words) - 08:46, 20 July 2024
Bent's rule (section Nonbonding orbitals)
atoms from the lower periods, trends in orbital hybridization depend strongly on both electronegativity and orbital size. In the early 1930s, shortly after...
38 KB (4,243 words) - 07:47, 11 June 2024
of organisms to create a hybrid Orbital hybridization, in chemistry, the mixing of atomic orbitals into new hybrid orbitals Nucleic acid hybridization...
1 KB (175 words) - 02:42, 28 February 2022
chemistry, the bonding orbital is used in molecular orbital (MO) theory to describe the attractive interactions between the atomic orbitals of two or more atoms...
9 KB (1,093 words) - 22:34, 24 September 2022
is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Each atomic orbital of an atom (specified by the three...
3 KB (420 words) - 09:22, 18 November 2023
plane of the ring in an sp2 hybrid orbital and does not participate in the conjugation. A requirement for conjugation is orbital overlap; thus, the conjugated...
35 KB (4,287 words) - 23:02, 29 July 2024
importance of orbital overlap in the molecular bond angles observed through experimentation; it is the basis for orbital hybridization. As s orbitals are spherical...
4 KB (509 words) - 01:19, 4 January 2024
An electron orbital may refer to: An atomic orbital, describing the behaviour of an electron in an atom A molecular orbital, describing the behaviour...
472 bytes (94 words) - 08:06, 27 October 2017
bond orbital or NBO is a calculated bonding orbital with maximum electron density. The NBOs are one of a sequence of natural localized orbital sets that...
5 KB (548 words) - 21:13, 16 October 2022
order hybridization is an extension of orbital hybridization, the mixing of atomic orbitals into hybrid orbitals which can form chemical bonds, to include...
7 KB (1,049 words) - 01:09, 5 February 2021
molecular orbital is essentially the carbon 1s atomic orbital, and the 2σ is the C–H bonding orbital formed by overlap of a carbon sp hybrid orbital with the...
6 KB (660 words) - 20:47, 22 October 2023
(σ(out)) is formed from a hybrid orbital that mixes 2s and 2p character, while the π-symmetry lone pair (p) is of exclusive 2p orbital parentage. The s character...
23 KB (2,947 words) - 11:17, 31 January 2024
orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of...
12 KB (1,576 words) - 07:10, 29 April 2024
Department Natural hybrid orbitals, one of a set of natural localized orbital sets in quantum chemistry, see Natural bond orbital Takuu language, a Polynesian...
708 bytes (127 words) - 03:47, 9 June 2024
In quantum mechanics, an atomic orbital (/ˈɔːrbɪtəl/) is a function describing the location and wave-like behavior of an electron in an atom. This function...
84 KB (10,932 words) - 22:39, 30 July 2024
an atom that employs an spx hybrid orbital for bonding will be more heavily polarized to that atom when the hybrid orbital has more s character. That is...
36 KB (4,507 words) - 20:10, 13 May 2024
atoms. The molecular orbital theory explanation (Bent's rule) is that lowering the energy of the oxygen atom's nonbonding hybrid orbitals (by assigning them...
88 KB (9,550 words) - 07:12, 7 August 2024
Albanese as Australia's first sovereign-made orbital rocket, is planned for no earlier than 2024 from the Bowen Orbital Spaceport in Abbot Point, Bowen. Gilmour...
20 KB (1,825 words) - 04:12, 24 June 2024
First and Second Orbital Wars, culminating in the corporate powers' complete takeover of the satellite collective of Earth's orbital belt. Upon the end...
9 KB (1,149 words) - 00:05, 24 July 2023
in a pure p orbital, another is in an spx hybrid orbital). The question of whether it is conceptually useful to derive equivalent orbitals from symmetry-adapted...
8 KB (938 words) - 21:41, 25 October 2023
shared electron (rather than two); in molecular orbital terms, the third electron is in an anti-bonding orbital which cancels out half of the bond formed by...
28 KB (3,654 words) - 07:13, 1 April 2024
fragments have similar frontier orbitals, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). Isolobal compounds...
10 KB (1,091 words) - 07:59, 7 March 2024
[Ne]3s23p2. Of these, four are valence electrons, occupying the 3s orbital and two of the 3p orbitals. Like the other members of its group, the lighter carbon and...
86 KB (10,663 words) - 03:23, 5 August 2024
electrons on P. A molecular orbital theory description considers the highest occupied molecular orbital to be a non-bonding orbital localized on the five fluorine...
22 KB (2,869 words) - 19:16, 11 May 2024
on valence bond theory. It is based on orbital strength functions, which are maximized when the hybrid orbitals on the atom are orthogonal[broken anchor]...
7 KB (1,245 words) - 18:52, 29 July 2024
two non-bonding orbitals, orbital mixing can be further performed between the 2p (3a1) orbital on oxygen and the antibonding 4a1 orbital since they are...
15 KB (1,822 words) - 11:34, 13 March 2023
and the bonds are called σ-bonds. The pz orbital is called a π-orbital. A π-bond occurs when two π-orbitals interact. This occurs when their nodal planes...
16 KB (2,235 words) - 20:52, 2 January 2024