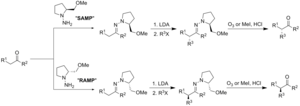
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates...
17 KB (1,800 words) - 14:54, 13 May 2023
The Stork enamine alkylation involves the addition of an enamine to a Michael acceptor (e.g., an α,β -unsaturated carbonyl compound) or another electrophilic...
3 KB (404 words) - 10:57, 12 February 2024
pattern. Non-carbon nucleophiles such as water, alcohols, amines, and enamines can also react with an α,β-unsaturated carbonyl in a 1,4-addition. Some...
27 KB (2,825 words) - 16:10, 22 November 2024
Shevchenko National University of Kyiv. He is also a scientific advisor of Enamine Ltd (Ukraine) and Lumobiotics GmbH (Germany). Source: Igor V. Komarov graduated...
24 KB (2,287 words) - 05:30, 12 November 2024
reactions. In 1963, G. Stork reported the first enamine alkylation reaction for ketones - Stork enamine alkylation reaction. In 1976, Meyers reported the...
23 KB (2,515 words) - 20:33, 22 July 2024
is widely used to convert ketones to enamines. Enamines derived from piperidine are substrates in the Stork enamine alkylation reaction. Upon treatment...
17 KB (1,471 words) - 14:44, 17 December 2024
aldehydes toward nucleophilic addition by formation of enamines (e.g. used in the Stork enamine alkylation): International Union of Pure and Applied Chemistry...
8 KB (589 words) - 14:40, 29 June 2023
titanium tetrachloride. In the Stork enamine alkylation, secondary amines form enamines when exposed to ketones. These enamines then react (possibly enantioselectively)...
40 KB (4,199 words) - 09:58, 30 November 2024
the modern field of asymmetric organocatalysis. Researches on asymmetric enamine catalysis applied to important intermediates in steroids synthesis is due...
22 KB (2,633 words) - 04:33, 13 April 2024
from o-nitrotoluenes 1.[1][2][3] The first step is the formation of an enamine 2 using N,N-dimethylformamide dimethyl acetal and pyrrolidine.[4] The desired...
4 KB (473 words) - 23:51, 31 August 2024
varying proportions. The active species is the hexafluoropropylamine; any enamine is converted into this by the hydrogen fluoride byproduct as the reaction...
2 KB (242 words) - 21:29, 15 April 2020
[3+3]cycloaddition between a cyclic enone and an enamine catalyzed by n-butyllithium is a Stork enamine / 1,2-addition cascade reaction: Iron[pyridine(diimine)]...
12 KB (1,317 words) - 23:37, 6 December 2024
bonded are far more common. Isothiazones are produced by oxidation of enamine-thiones. The ring structure of isothiazole is incorporated into larger...
3 KB (181 words) - 21:07, 29 October 2023
of proline in the transition state determine the reaction outcome. An enamine is formed during the reaction and only one proline molecule is involved...
20 KB (1,841 words) - 15:30, 19 May 2024
ammonia or a primary amine gives an imine With secondary amine gives an enamine With Grignard and organolithium reagents to give, after aqueous workup...
24 KB (2,895 words) - 03:58, 25 November 2024
Nitrogen Amine Enamine Ammonium Hydrazo Nitrene Imine Oxime Hydrazone Azo Amide Imidate Amidine Carbamate Imide Nitrile Isonitrile Cyanate Isocyanate...
91 KB (9,591 words) - 14:42, 4 January 2025
reason, it forms a stable chloramine. It is commonly used to generate enamines. Morpholine is widely used in organic synthesis. For example, it is a building...
10 KB (786 words) - 00:52, 30 June 2024
Nitrogen Amine Enamine Ammonium Hydrazo Nitrene Imine Oxime Hydrazone Azo Amide Imidate Amidine Carbamate Imide Nitrile Isonitrile Cyanate Isocyanate...
26 KB (2,619 words) - 12:07, 27 December 2024
Nitrogen Amine Enamine Ammonium Hydrazo Nitrene Imine Oxime Hydrazone Azo Amide Imidate Amidine Carbamate Imide Nitrile Isonitrile Cyanate Isocyanate...
61 KB (7,066 words) - 03:47, 3 January 2025
and reduction by dihydroprecondylocarpine acetate synthase (DPAS), an enamine intermediate is formed. The intermediate undergoes fragmentation to produce...
62 KB (6,238 words) - 03:22, 20 December 2024
Nitrogen Amine Enamine Ammonium Hydrazo Nitrene Imine Oxime Hydrazone Azo Amide Imidate Amidine Carbamate Imide Nitrile Isonitrile Cyanate Isocyanate...
19 KB (1,843 words) - 18:54, 16 December 2024
Nitrogen Amine Enamine Ammonium Hydrazo Nitrene Imine Oxime Hydrazone Azo Amide Imidate Amidine Carbamate Imide Nitrile Isonitrile Cyanate Isocyanate...
14 KB (1,505 words) - 19:27, 12 December 2024
is a form of inverse-electron demand Diels-Alder reaction in which an enamine reacts with a 1,2,4-triazine to form the pyridine nucleus. The reaction...
2 KB (266 words) - 18:45, 28 July 2016
Nitrogen Amine Enamine Ammonium Hydrazo Nitrene Imine Oxime Hydrazone Azo Amide Imidate Amidine Carbamate Imide Nitrile Isonitrile Cyanate Isocyanate...
46 KB (4,846 words) - 04:43, 20 November 2024
pairs include: ketone – enol: H−O−C=C ⇌ O=C−C−H, see keto–enol tautomerism enamine – imine: H−N−C=C ⇌ N=C−C−H cyanamide – carbodiimide guanidine – guanidine...
14 KB (1,492 words) - 15:12, 5 December 2024
Nitrogen Amine Enamine Ammonium Hydrazo Nitrene Imine Oxime Hydrazone Azo Amide Imidate Amidine Carbamate Imide Nitrile Isonitrile Cyanate Isocyanate...
62 KB (6,986 words) - 13:34, 25 December 2024
ketone) initially forms a phenylhydrazone which isomerizes to the respective enamine (or 'ene-hydrazine'). After protonation, a cyclic [3,3]-sigmatropic rearrangement...
9 KB (793 words) - 14:46, 26 September 2024
electron delocalisation with the indole ring.[citation needed] LSD also has enamine-type reactivity because of the electron-donating effects of the indole...
179 KB (17,621 words) - 22:43, 4 January 2025
subsequent hydrolysis of the remaining aluminium and iron. Acetals, imines, and enamines can be converted back into ketones by treatment with excess water under...
18 KB (2,238 words) - 16:16, 1 November 2024
Nitrogen Amine Enamine Ammonium Hydrazo Nitrene Imine Oxime Hydrazone Azo Amide Imidate Amidine Carbamate Imide Nitrile Isonitrile Cyanate Isocyanate...
9 KB (1,047 words) - 12:55, 17 December 2024