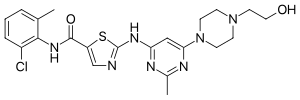
Imatinib, sold under the brand names Gleevec and Glivec (both marketed worldwide by Novartis) among others, is an oral targeted therapy medication used...
72 KB (6,714 words) - 03:57, 10 February 2025
Chronic myelogenous leukemia (section Imatinib)
action as a tyrosine kinase, targeted therapies (the first of which was imatinib) that specifically inhibit the activity of the BCR-ABL protein have been...
34 KB (3,877 words) - 13:44, 25 October 2024
GlaxoSmithKline in 2015 deal), carbamazepine (Tegretol), valsartan (Diovan), imatinib mesylate (Gleevec/Glivec), cyclosporine (Neoral/Sandimmune), letrozole...
153 KB (12,596 words) - 22:39, 10 March 2025
patent imatinib mesylate in beta crystalline form (rather than imatinib or imatinib mesylate); thus they sought to prevent extant literature on imatinib or...
43 KB (5,300 words) - 23:28, 21 October 2024
exon 17 are responsible for resistance to targeted therapy drugs like imatinib mesylate, a tyrosine kinase inhibitor. KIT-p.D419del (exon 8) — A subset...
46 KB (4,770 words) - 17:16, 23 February 2025
kinase. Before the 2001 U.S. Food and Drug Administration (FDA) approval of imatinib, no drugs were available to alter the natural progression of CML. Only...
56 KB (6,470 words) - 20:27, 19 November 2024
anion, the spelling used is sometimes mesilate (as in imatinib mesilate, the mesylate salt of imatinib). Mesylate esters are a group of organic compounds...
4 KB (426 words) - 17:55, 25 November 2024
tissue. One of the most successful molecular targeted therapeutics is imatinib, marketed as Gleevec, which is a kinase inhibitor with exceptional affinity...
20 KB (2,244 words) - 17:15, 4 February 2025
Tyrosine kinase (section GIST and Imatinib)
step for the development of cancer. Therefore, kinase inhibitors, such as imatinib and osimertinib, are often effective cancer treatments. Most tyrosine kinases...
44 KB (5,283 words) - 12:50, 27 December 2024
small number of children also develop this disease. It is treated with imatinib (Gleevec in United States, Glivec in Europe) or other drugs. The five-year...
83 KB (8,178 words) - 01:01, 26 February 2025
reactions with phenelzine, and a potential interaction has been reported with imatinib, resulting in hepatotoxicity, and with lamotrigine. The common ginsengs...
36 KB (3,655 words) - 04:06, 17 February 2025
the years. However, French scientists treating leukemia patients with imatinib, a drug used in treating cancer, noted an unexpected side effect: some...
17 KB (1,947 words) - 22:31, 26 February 2025
well as in accelerated and chronic phase CML that has not responded to imatinib. It is taken by mouth. Common side effects may include low platelets, low...
24 KB (1,935 words) - 22:20, 10 March 2025
approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) in January 2006. Sunitinib...
36 KB (3,366 words) - 03:36, 12 March 2025
cancer's cell division. The classic example of targeted development is imatinib mesylate (Gleevec), a small molecule which inhibits a signaling molecule...
31 KB (3,847 words) - 14:06, 16 February 2025
CML CP) or Ph+ CML CP resistant or intolerant to prior therapy including imatinib. newly diagnosed Ph+ acute lymphoblastic leukaemia (ALL) in combination...
22 KB (1,994 words) - 21:53, 8 March 2025
kinase inhibitors specific to such domains as CC, Y177, and Rho (such as imatinib and sunitinib) are important drugs against a variety of cancers including...
29 KB (3,538 words) - 15:01, 5 December 2023
Topotecan Etoposide Teniposide Tafluposide Bortezomib Erlotinib Gefitinib Imatinib Vemurafenib Vismodegib Azacitidine Azathioprine Capecitabine Cladribine...
5 KB (324 words) - 08:56, 9 December 2024
to imatinib. However, if the mutation occurs in exon 17 (as is often the case in seminomas and leukemias), the receptor is not inhibited by imatinib. In...
35 KB (4,117 words) - 00:12, 6 August 2024
Fluvoxamine (Luvox, Faverin, Fevarin and Dumyrox) Imatinib (Gleevec): Although no formal studies with imatinib and grapefruit juice have been conducted, the...
55 KB (5,694 words) - 09:02, 14 February 2025
is now routine practice, as its presence indicates a likely response to imatinib, a tyrosine kinase inhibitor. Chusid et al. developed empirical diagnostic...
26 KB (2,491 words) - 20:07, 10 September 2024
US FDA approved (imatinib mesylate) for the treatment of DFSP. As is true for all medicinal drugs with name ending in "ib," imatinib is a small molecular...
29 KB (3,146 words) - 07:05, 2 December 2024
return for a large sum of money. Due to patent protection, the Swiss drug imatinib is very expensive and cannot be afforded by most leukemia patients in China...
10 KB (798 words) - 11:06, 21 September 2024
rituximab, sirolimus, alefacept, and the tyrosine kinase inhibitors, imatinib, nilotinib, and dasatinib. Experimental therapies under investigation include...
38 KB (3,337 words) - 22:36, 4 January 2025
γ-secretase. Imatinib itself does not get into the brain so imatinib could not be used as an AD therapeutic. However it may be possible to identify imatinib-like...
8 KB (819 words) - 00:00, 4 March 2023
protein can be inhibited by various small molecules. One such inhibitor is imatinib mesylate, which occupies the tyrosine kinase domain and inhibits BCR-ABL's...
30 KB (3,502 words) - 08:07, 14 August 2024
that have the T315I mutation, are resistant to current therapies such as imatinib. Ponatinib has been designed to be effective against these types of tumors...
18 KB (1,425 words) - 05:43, 20 February 2025
effective anti-tumor agents and anti-leukemic agents. Based on this work imatinib was developed against chronic myelogenous leukemia (CML) and later gefitinib...
10 KB (1,179 words) - 18:31, 8 August 2024
Dean for Oncology in the OHSU School of Medicine. Druker helped develop imatinib (Gleevec), the first medication that specifically targets cancer cells...
27 KB (2,205 words) - 00:17, 28 January 2025
Charlotte F, Salvatierra J, Wechsler B, Graux C, et al. (June 2008). "Imatinib mesylate for platelet-derived growth factor receptor-beta-positive Erdheim-Chester...
22 KB (2,230 words) - 07:46, 26 November 2024