12-Crown-4
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1,4,7,10-Tetraoxacyclododecane | |||
| Other names 12-crown-4, Lithium Ionophore V | |||
| Identifiers | |||
3D model (JSmol) | |||
| 1363064 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.488 | ||
| EC Number |
| ||
| 3287 | |||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C8H16O4 | |||
| Molar mass | 176.21 | ||
| Density | 1.089 g/mL at 25 °C | ||
| Melting point | 16 °C | ||
| Boiling point | 61-70 °C/0.5 mm Hg | ||
| Miscible | |||
| Hazards | |||
| Flash point | 113 °C (235 °F; 386 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
12-Crown-4, also called 1,4,7,10-tetraoxacyclododecane and lithium ionophore V, is a crown ether with the formula C8H16O4. It is a cyclic tetramer of ethylene oxide which is specific for the lithium cation.
Synthesis
[edit]12-Crown-4 can be synthesized using a modified Williamson ether synthesis, using LiClO4 as a templating cation:[1]
- (CH2OCH2CH2Cl)2 + (CH2OH)2 + 2 NaOH → (CH2CH2O)4 + 2 NaCl + 2 H2O
It also forms from the cyclic oligomerization of ethylene oxide in the presence of gaseous boron trifluoride.[2]
Properties
[edit]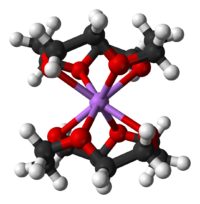
Like other crown ethers, 12-crown-4 complexes with alkali metal cations. The cavity diameter of 1.2-1.5 Å gives it a high selectivity towards the lithium cation (ionic diameter 1.36 Å)[2]
Its point group is S4. The dipole moment of 12-crown-4 varies with solvent and temperature. At 25 °C, the dipole moment of 12-crown-4 was determined as 2.33 ± 0.03 D in cyclohexane and 2.46 ± 0.01 D in benzene.[3]
References
[edit]- ^ Cook, Fred L.; Caruso, Thomas C.; Byrne, Michael P.; Bowers, Chauncey W.; Speck, Don H.; Liotta, Charles L. (1974). "Facile syntheses of 12-crown-4 and 15-crown-5". Tetrahedron Letters. 15 (46): 4029–4032. doi:10.1016/S0040-4039(01)92075-1.
- ^ a b Liotta, Charles L.; Berkner, Joachim (2001), "12-Crown-4", Encyclopedia of Reagents for Organic Synthesis, Chichester, UK: John Wiley & Sons, Ltd, doi:10.1002/047084289X.rc262, ISBN 978-0-471-93623-7
- ^ Caswell, Lyman R.; Savannunt, Diana S. (January 1988). "Temperature and solvent effects on the experimental dipole moments of three crown ethers". Journal of Heterocyclic Chemistry. 25 (1): 73–79. doi:10.1002/jhet.5570250111.
- Sigma-Aldrich Handbook of Fine Chemicals, 2007, page 768.
- Sigma-Aldrich Cyclic tetramer of ethylene oxide which is specific for the lithium cation. 98%, 2018
See also
[edit]- Crown ether
- Cyclen, a similar molecule with N atoms (aza groups) instead of O atoms (ethers)


 French
French Deutsch
Deutsch