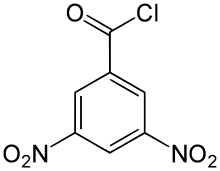
3,5-Dinitrobenzoyl chloride
 3,5-Dinitrobenzoyl chloride | |
| Names | |
|---|---|
| Preferred IUPAC name 3,5-Dinitrobenzoyl chloride | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.500 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C7H3ClN2O5 | |
| Molar mass | 230.56 g·mol−1 |
| Melting point | 68–69 °C (154–156 °F; 341–342 K) |
| Boiling point | 196 °C (385 °F; 469 K) 11 mmHg |
| Hazards | |
| GHS labelling: | |
 | |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
3,5-Dinitrobenzoyl chloride (C7H3ClN2O5) is an organic compound with a melting point of 68–69 °C.[1] It is the acyl chloride of 3,5-dinitrobenzoic acid and like it is mainly used in the analysis of organic substances by derivatization.[2]
Synthesis
[edit]3,5-dinitrobenzoyl chloride is prepared by the reaction of 3,5-dinitrobenzoic acid with phosphorus pentachloride, PCl5.[3] It can likewise be prepared by reaction with phosphorus trichloride, PCl3, or thionyl chloride, SOCl2.[4]
Uses
[edit]3,5-Dinitrobenzoyl chloride has its main use in the analysis of organic compounds by derivatization, specifically of alcohols and amines. It is used in cases where the substance to be analyzed is sensitive and cannot be directly reacted with 3,5-dinitrobenzoic acid. Generally, the reaction is done in pyridine in order to bind the hydrogen chloride released.[5] With this method, it is for instance possible to identify amino acids.[3]

Identification of alanine via reaction with 3,5-dinitrobenzoyl chloride. The product has a melting point of 177 °C[6]
References
[edit]- ^ Sigma-Aldrich Co., 3,5-Dinitrobenzoyl chloride. Retrieved on 12. März 2017.
- ^ W. T. Robinson, R. H. Cundiff, P. C. Markunas: „Rapid Determination of Organic Hydroxyl Groups with 3,5-Dinitrobenzoyl Chloride“, in: Anal. Chem., 1961, 33 (8), S. 1030–1034 (doi:10.1021/ac60176a050).
- ^ a b Saunders, B. C.; Stacey, G. J.; Wilding, I. G. E. (1942). "The Preparation of 3:5-Dinitrobenzoic Acid and 3:5-Dinitrobenzoyl Chloride – Observations on the Acylation of Amino-acids by means of 3:5-Dinitrobenzoyl Chloride and certain other Acid Chlorides". Biochem. J. 36 (3–4): 368–375. doi:10.1042/bj0360368. PMC 1265703. PMID 16747534.
- ^ Organikum (in German) (19th ed.). Leipzig·Berlin·Heidelberg: Johann Ambrosius Barth. 1993. p. 440. ISBN 3335003438.
- ^ Organikum (in German) (19th ed.). Leipzig·Berlin·Heidelberg: Johann Ambrosius Barth. 1993. p. 424. ISBN 3335003438.
- ^ CRC Handbook of Tables for Organic Compound Identification, Third Edition, 1984, ISBN 0-8493-0303-6.


 French
French Deutsch
Deutsch
