Brodimoprim
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.054.745 |
| Chemical and physical data | |
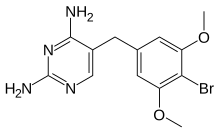
| Formula | C13H15BrN4O2 |
| Molar mass | 339.193 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 225 to 228 °C (437 to 442 °F) |
| |
| |
| (verify) | |
Brodimoprim is a structural derivative of trimethoprim. In brodimoprim, the 4-methoxy group of trimethoprim is replaced with a bromine atom.
As trimethoprim, brodimoprim is a selective inhibitor of bacterial dihydrofolate reductase.[1]
Synthesis
[edit]
The treatment of Dimethyl 2,6-dimethoxybenzene-1,4-dicarboxylate [16849-68-6] (1) with hydroxylamine in PPA gives the hydroxamide, PC12398304 (2). Further treatment with PPA led to methyl 4-amino-3,5-dimethoxybenzoate [56066-25-2] (3). Sandmeyer reaction led to Methyl 4-bromo-3,5-dimethoxybenzoate [26050-64-6] (4). Saponification of the ester formed 4-Bromo-3,5-dimethoxybenzoic acid [56518-42-4] (5). Halogenation with thionyl chloride gave 4-Bromo-3,5-dimethoxybenzoyl chloride [56518-43-5] (6). Rosenmund reduction gave 4-Bromo-3,5-dimethoxybenzaldehyde [31558-40-4] (7). {Alternatively DIBAL meant that FGI from ester to aldehyde was accomplished in only 1 step}. Knoevenagel condensation with 3-Methoxypropionitrile [110-67-8] (8) afforded [56518-39-9] (9). Finally, condensation with Guanidine [113-00-8] completed the synthesis of Brodimoprim (10).
References
[edit]- ^ Thomson CJ (December 1993). "Trimethoprim and brodimoprim resistance of gram-positive and gram-negative bacteria". Journal of Chemotherapy. 5 (6): 458–64. doi:10.1080/1120009X.1993.11741096. PMID 8195838.
- ^ Sweetman, AJ; Serradell, MN; Castaer, J.; Blancafort, P.; Brodimoprim. Drugs Fut 1982, 7, 2, 93.
- ^ Kompis, Ivan; Wick, Alexander (1977). "Synthese von 4-halogensubstituierten Analogen von Trimethoprim". Helvetica Chimica Acta. 60 (8): 3025–3034. doi:10.1002/hlca.19770600854.
- ^ Max Dr. Nutley N.J. Us Hoffer, Ivan Dr. Oberwil Ch Kompis, DE2452889 (1985 to F. Hoffmann-La Roche & Co Ag, Basel, Ch); CA, 83, 9736lt
- ^ Barfknecht, C. F., Nichols, D. E. (April 1971). "Potential psychotomimetics. Bromomethoxyamphetamines". Journal of Medicinal Chemistry. 14 (4): 370–372. doi:10.1021/jm00286a026.


 French
French Deutsch
Deutsch