Cyclooctatetraene
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Cycloocta-1,3,5,7-tetraene[1] | |||
| Other names [8]Annulene (1Z,3Z,5Z,7Z)-Cycloocta-1,3,5,7-tetraene 1,3,5,7-Cyclooctatetraene COT | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.010.074 | ||
| EC Number |
| ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C8H8 | |||
| Molar mass | 104.15 g/mol | ||
| Appearance | Clear yellow | ||
| Density | 0.9250 g/cm3, liquid | ||
| Melting point | −5 to −3 °C (23 to 27 °F; 268 to 270 K) | ||
| Boiling point | 142 to 143 °C (288 to 289 °F; 415 to 416 K) | ||
| immiscible | |||
| -53.9·10−6 cm3/mol | |||
| Hazards | |||
| GHS labelling: | |||
   | |||
| Danger | |||
| H225, H304, H315, H319, H335 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+P310, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P312, P321, P331, P332+P313, P337+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −11 °C (12 °F; 262 K) | ||
| 561 °C (1,042 °F; 834 K) | |||
| Related compounds | |||
Related hydrocarbons | Cyclooctane Tetraphenylene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy.
Unlike benzene, C6H6, cyclooctatetraene, C8H8, is not aromatic, although its dianion, C
8H2−
8 (cyclooctatetraenide), is. Its reactivity is characteristic of an ordinary polyene, i.e. it undergoes addition reactions. Benzene, by contrast, characteristically undergoes substitution reactions, not additions.
History
[edit]1,3,5,7-Cyclooctatetraene was initially synthesized by Richard Willstätter in Munich in 1905 using pseudopelletierine as the starting material and the Hofmann elimination as the key transformation:[2][3]
Willstätter noted that the compound did not exhibit the expected aromaticity. Between 1939 and 1943, chemists throughout the US unsuccessfully attempted to synthesize COT. They rationalized their lack of success with the conclusion that Willstätter had not actually synthesized the compound but instead its isomer, styrene. Willstätter responded to these reviews in his autobiography, where he noted that the American chemists were 'untroubled' by the reduction of his cyclooctatetraene to cyclooctane (a reaction impossible for styrene). During World War II, Walter Reppe at BASF Ludwigshafen developed a simple, one-step synthesis of cyclooctatetraene from acetylene, providing material identical to that prepared by Willstätter.[4] Any remaining doubts on the accuracy of Willstätter's original synthesis were resolved when Arthur C. Cope and co-workers at MIT reported, in 1947, a complete repetition of the Willstätter synthesis, step by step, using the originally reported techniques. They obtained the same cyclooctatetraene,[5] and they subsequently reported modern spectral characterization of many of the intermediate products, again confirming the accuracy of Willstätter's original work.[6] However, the freezing temperature of the product was different from pure COT, and the authors interpreted it as contamination with about 30% of styrene.
Structure and bonding
[edit]
Early studies demonstrated that COT did not display the chemistry of an aromatic compound.[7] Then, early electron diffraction experiments concluded that the C-C bond distances were identical.[8] However, X-ray diffraction data from H. S. Kaufman demonstrated cyclooctatetraene to adopt several conformations and to contain two distinct C–C bond distances.[9] This result indicated that COT is an annulene with fixed alternating single and double C-C bonds.
In its normal state, cyclooctatetraene is non-planar and adopts a tub conformation with angles C=C−C = 126.1° and C=C−H = 117.6°.[10] The point group of cyclooctatetraene is D2d.[11]
In its planar transition state, the D4h transitional state is more stable than the D8h transitional state due to the Jahn–Teller effect.[12]
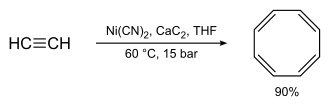
Synthesis
[edit]Richard Willstätter's original synthesis (4 consecutive elimination reactions on a cyclooctane framework) gives relatively low yields. Reppe's synthesis of cyclooctatetraene, which involves treating acetylene at high pressure with a warm mixture of nickel cyanide and calcium carbide, was much better, with chemical yields near 90%:[4]
COT can also be prepared by photolysis of barrelene, one of its structural isomers, the reaction proceeding via another isolable isomer, semibullvalene.[13] COT derivatives can also be synthesised by way of semibullvalene intermediates. In the sequence illustrated below, octaethylcyclooctatetraene (C8Et8) is formed by thermal isomerisation of octaethylsemibullvalene, itself formed by copper(I) bromide mediated cyclodimerisation of 1,2,3,4-tetraethyl-1,4-dilithio-1,3-butadiene.[14]
Because COT is unstable and easily forms explosive organic peroxides, a small amount of hydroquinone is usually added to commercially available material. Testing for peroxides is advised when using a previously opened bottle; white crystals around the neck of the bottle may be composed of the peroxide, which may explode when mechanically disturbed.
Natural occurrence
[edit]Cyclooctatetraene has been isolated from certain fungi.[15]
Reactions
[edit]The π bonds in COT react as usual for olefins, rather than as aromatic ring systems. Mono- and polyepoxides can be generated by reaction of COT with peroxy acids or with dimethyldioxirane. Various other addition reactions are also known. Furthermore, polyacetylene can be synthesized via the ring-opening polymerization of cyclooctatetraene.[16] COT itself—and also analogs with side-chains—have been used as metal ligands and in sandwich compounds.
Cyclooctatetraene also undergoes rearrangement reactions to form aromatic ring systems. For instance, oxidation with aqueous mercury(II) sulfate forms phenylacetaldehyde[4][17] and photochemical rearrangement of its monoepoxide forms benzofuran.[18]
Cyclooctatetraenide as a ligand and ligand precursor
[edit]
COT readily reacts with potassium metal to form the salt K2COT, which contains the dianion C
8H2−
8.[19] The dianion is planar, octagonal, and aromatic with a Hückel electron count of 10.
Cyclooctatetraene forms organometallic complexes with some metals, including yttrium, lanthanides, and actinides.[20] The sandwich compound uranocene (U(COT)2) features two η8-COT ligands. In bis(cyclooctatetraene)iron (Fe(COT)2) one COT is η6 and the other is η4. (Cyclooctatetraene)iron tricarbonyl features η4-COT. The room-temperature 1H NMR spectra of these iron complexes are singlets, indicative of fluxionality.[21]
Cyclooctatetraene is chlorinated to give a [4.2.0]-bicyclic compound, which reacts further with dimethyl acetylenedicarboxylate in a Diels-Alder reaction (DA). Retro-DA at 200 °C releases cis-dichlorocyclobutene. This compound reacts with diiron nonacarbonyl to give cyclobutadieneiron tricarbonyl.[22][23]
See also
[edit]- Barrelene, structural isomer of cyclooctatetraene
- Benzene
- Cyclobutadiene
- Heptafulvene, structural isomer of cyclooctatetraene
- Pentalene
- Semibullvalene
References
[edit]- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. P001 – P004. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Mason, S. (February 1997). "The Science and Humanism of Linus Pauling (1901−1994)". Chem. Soc. Rev. 26: 29–39. doi:10.1039/CS9972600029.
- ^ Richard Willstätter; Ernst Waser (1911). "Über Cyclo-octatetraen" [On cyclooctatetraene]. Berichte der Deutschen Chemischen Gesellschaft. 44 (3): 3423–3445. doi:10.1002/cber.191104403216.
- ^ a b c Reppe, Walter; Schlichtin, Otto; Klager, Karl; Toepel, Tim (1948). "Cyclisierende Polymerisation von Acetylen. I. Über Cyclooctatetraen" [Ring-forming polymerization of acetylene. I. Cyclooctatetraene]. Justus Liebigs Annalen der Chemie. 560 (1): 1–92. doi:10.1002/jlac.19485600102.
- ^ Cope, Arthur C.; Overberger, C. G. (1947). "The synthesis of cycloöctatetraene from pseudopelletierine". Journal of the American Chemical Society. 69 (4): 976. doi:10.1021/ja01196a513. PMID 20292490.
- ^ Cope, Arthur C.; Overberger, C. G. (1947). "Cyclic Polyolefins. I. Synthesis of Cycloöctatetraene from Pseudopelletierine". Journal of the American Chemical Society. 70 (4): 1433–1437. doi:10.1021/ja01184a041. PMID 18915758.
- ^ Johnson, A. W. (1947). "Organic Chemistry". Sci. Progr. 35 (139): 506–515. JSTOR 43413011.
- ^ Bastiensen, O.; Hassel, O.; Langseth, A. (1947). "The 'Octa-Benzene', Cyclo-octatetraene (C8H8)". Nature. 160 (4056): 128. Bibcode:1947Natur.160..128B. doi:10.1038/160128a0.
- ^ Kaufman, H. S.; Fankuchen, I.; H., Mark (1948). "Structure of Cyclo-octatetraene". Nature. 161 (4083): 165. Bibcode:1948Natur.161..165K. doi:10.1038/161165a0.
- ^ Thomas, P. M.; Weber, A. (1978). "High resolution Raman spectroscopy of gases with laser sources. XIII – the pure rotational spectra of 1,3,5,7-cyclooctatetraene and 1,5-cyclooctadiene". J. Raman Spectr. 7 (6): 353–357. Bibcode:1978JRSp....7..353T. doi:10.1002/jrs.1250070614.
- ^ Claus, K. H.; Krüger, C. (15 September 1988). "Structure of cyclooctatetraene at 129 K". Acta Crystallogr. C. 44 (9): 1632–1634. Bibcode:1988AcCrC..44.1632C. doi:10.1107/S0108270188005840.
- ^ Nishinaga, Tohru; Ohmae, Takeshi; Iyoda, Masahiko (5 February 2010). "Recent Studies on the Aromaticity and Antiaromaticity of Planar Cyclooctatetraene". Symmetry. 2 (1): 76–97. Bibcode:2010Symm....2...76N. doi:10.3390/sym2010076.
- ^ Zimmerman, H. E.; Grunewald, G. L. (1966). "The Chemistry of Barrelene. III. A Unique Photoisomerization to Semibullvalene". J. Am. Chem. Soc. 88 (1): 183–184. doi:10.1021/ja00953a045.
- ^ Wang, C.; Yuan, J.; Li, G.; Wang, Z.; Zhang, S.; Xi, Z. (2006). "Metal-Mediated Efficient Synthesis, Structural Characterization, and Skeletal Rearrangement of Octasubstituted Semibullvalenes". J. Am. Chem. Soc. 128 (14): 4564–4565. doi:10.1021/ja0579208. PMID 16594680.
- ^ Stinson, M.; Ezra, D.; Hess, W. M.; Sears, J.; Strobel, G. (2003). "An endophytic Gliocladium sp. of Eucryphia cordifolia producing selective volatile antimicrobial compounds". Plant Sci. 165 (4): 913–922. Bibcode:2003PlnSc.165..913S. doi:10.1016/S0168-9452(03)00299-1.
- ^ Moorhead, Eric J.; Wenzel, Anna G. (August 2009). "Two Undergraduate Experiments in Organic Polymers: The Preparation of Polyacetylene and Telechelic Polyacetylene via Ring-Opening Metathesis Polymerization". Journal of Chemical Education. 86 (8): 973. Bibcode:2009JChEd..86..973M. doi:10.1021/ed086p973.
- ^ Kunichika, Sango (1953). "Cyclopolyolefins Derived from Acetylene". Bulletin of the Institute for Chemical Research, Kyoto University. 31 (5): 323–335. hdl:2433/75368.
- ^ Holovka, J. M.; Gardner, P. D.; Strow, C. B.; Hill, M. L.; Van Auken, T. V. (1968). "Photolysis and photoisomerization of cyclooctatetraene oxide". Journal of the American Chemical Society. 90 (18): 5041–5043. doi:10.1021/ja01020a058.
- ^ Katz, Thomas J. (1960). "The cyclooctatetraenyl dianion". J. Am. Chem. Soc. 82 (14): 3784–3785. doi:10.1021/ja01499a077.
- ^ "JST Nanostructed Materials Project Highlights – Prof. Nakajima's Presentation". Archived from the original on 2008-02-19. Retrieved 2005-11-24.
- ^ Cotton, F. Albert; Hunter, Douglas L. (1976). "Carbon-13 Nuclear Magnetic Resonance Study of the Fluxional Behavior of Cyclooctatetraenetricarbonyliron and -Ruthenium". Journal of the American Chemical Society. 98 (6): 1413–1417. doi:10.1021/ja00422a022.
- ^ R. Pettit and J. Henery (1970). "cis-dichlorocyclobutene". Organic Syntheses. 50: 36. doi:10.15227/orgsyn.050.0036.
- ^ "CYCLOBUTADIENE IN SYNTHESIS: Endo-TRICYCLO[4.4.0.02,5]DECA-3,8-DIENE-7,10-DIONE". Organic Syntheses. 55: 43. 1976. doi:10.15227/orgsyn.055.0043.


 French
French Deutsch
Deutsch