Alzheimer's disease
| Alzheimer's disease | |
|---|---|
| Other names | Alzheimer's dementia |
 | |
| Diagram of a normal brain compared to the brain of a person with Alzheimer's | |
| Pronunciation |
|
| Specialty | Neurology |
| Symptoms | Memory loss, problems with language, disorientation, mood swings[1][2] |
| Complications | Infections, falls and aspiration pneumonia in the terminal stage[3] |
| Usual onset | Over 65 years old[4] |
| Duration | Long term[2] |
| Causes | Poorly understood[1] |
| Risk factors | Genetics, head injuries, clinical depression, hypertension,[1] psychological stress,[5] lack of physical[6] and mental[5][7] exercise |
| Diagnostic method | Based on symptoms and cognitive testing after ruling out other possible causes[8] |
| Differential diagnosis | Normal brain aging,[1] Lewy body dementia,[9] Trisomy 21[10] |
| Medication | Acetylcholinesterase inhibitors, NMDA receptor antagonists[11] |
| Prognosis | Life expectancy 3–12 years[11][12][13] |
| Frequency | 50 million (2020)[14] |
| Named after | Alois Alzheimer |
Alzheimer's disease (AD) is a neurodegenerative disease that usually starts slowly and progressively worsens,[2] and is the cause of 60–70% of cases of dementia.[2][15] The most common early symptom is difficulty in remembering recent events.[1] As the disease advances, symptoms can include problems with language, disorientation (including easily getting lost), mood swings, loss of motivation, self-neglect, and behavioral issues.[2] As a person's condition declines, they often withdraw from family and society.[16] Gradually, bodily functions are lost, ultimately leading to death. Although the speed of progression can vary, the average life expectancy following diagnosis is three to twelve years.[11][12][13]
The cause of Alzheimer's disease is poorly understood.[16] There are many environmental and genetic risk factors associated with its development. The strongest genetic risk factor is from an allele of apolipoprotein E.[17][18] Other risk factors include a history of head injury, clinical depression, and high blood pressure.[1] The progression of the disease is largely characterized by the accumulation of malformed protein deposits in the cerebral cortex, called amyloid plaques and neurofibrillary tangles. These misfolded protein aggregates interfere with normal cell function, and over time lead to irreversible degeneration of neurons and loss of synaptic connections in the brain.[19] A probable diagnosis is based on the history of the illness and cognitive testing, with medical imaging and blood tests to rule out other possible causes.[8][20] Initial symptoms are often mistaken for normal brain aging.[16] Examination of brain tissue is needed for a definite diagnosis, but this can only take place after death.[21][22]
No treatments can stop or reverse its progression, though some may temporarily improve symptoms.[2] A healthy diet, physical activity, and social engagement are generally beneficial in aging, and may help in reducing the risk of cognitive decline and Alzheimer's.[19] Affected people become increasingly reliant on others for assistance, often placing a burden on caregivers.[23] The pressures can include social, psychological, physical, and economic elements.[23] Exercise programs may be beneficial with respect to activities of daily living and can potentially improve outcomes.[24] Behavioral problems or psychosis due to dementia are sometimes treated with antipsychotics, but this has an increased risk of early death.[25][26]
As of 2020, there were approximately 50 million people worldwide with Alzheimer's disease.[14] It most often begins in people over 65 years of age, although up to 10% of cases are early-onset impacting those in their 30s to mid-60s.[27][4] It affects about 6% of people 65 years and older,[16] and women more often than men.[28] The disease is named after German psychiatrist and pathologist Alois Alzheimer, who first described it in 1906.[29] Alzheimer's financial burden on society is large, with an estimated global annual cost of US$1 trillion.[14] It is ranked as the seventh leading cause of death worldwide.[30]
Given the widespread impacts of Alzheimer's disease, both basic-science and health funders in many countries support Alzheimer's research at large scales. For example, the US National Institutes of Health program for Alzheimer's research, the National Plan to Address Alzheimer’s Disease, has a budget of US$3.98 billion for fiscal year 2026.[31] In the European Union, the 2020 Horizon Europe research programme awarded over €570 million for dementia-related projects.[32]
Signs and symptoms
The course of Alzheimer's is generally described in three stages, with a progressive pattern of cognitive and functional impairment.[33][27] The three stages are described as early or mild, middle or moderate, and late or severe.[33] The disease is known to target the hippocampus which is associated with memory, and this is responsible for the first symptoms of memory impairment. As the disease progresses so does the degree of memory impairment.[19]
First symptoms

The first symptoms are often mistakenly attributed to aging or stress.[34] Detailed neuropsychological testing can reveal mild cognitive difficulties up to eight years before a person fulfills the clinical criteria for diagnosis of Alzheimer's disease.[35] These early symptoms can affect the most complex activities of daily living.[36] The most noticeable deficit is short term memory loss, which shows up as difficulty in remembering recently learned facts and inability to acquire new information.[35]
Subtle problems with the executive functions of attentiveness, planning, flexibility, and abstract thinking, or impairments in semantic memory (memory of meanings, and concept relationships) can also be symptomatic of the early stages of Alzheimer's disease.[35] Apathy and depression can be seen at this stage, with apathy remaining as the most persistent symptom throughout the course of the disease.[37][38] Mild cognitive impairment (MCI) is often found to be a transitional stage between normal aging and dementia. MCI can present with a variety of symptoms, and when memory loss is the predominant symptom, it is termed amnestic MCI and is frequently seen as a prodromal stage of Alzheimer's disease.[39] Amnesic MCI has a greater than 90% likelihood of being associated with Alzheimer's.[40]
Early stage
In people with Alzheimer's disease, the increasing impairment of learning and memory eventually leads to a definitive diagnosis. In a small percentage, difficulties with language, executive functions, perception (agnosia), or execution of movements (apraxia) are more prominent than memory problems.[41] Alzheimer's disease does not affect all memory capacities equally. Older memories of the person's life (episodic memory), facts learned (semantic memory), and implicit memory (the memory of the body on how to do things, such as using a fork to eat or how to drink from a glass) are affected to a lesser degree than new facts or memories.[42][43]
Language problems are mainly characterised by a shrinking vocabulary and decreased word fluency, leading to a general impoverishment of oral and written language.[41][44] In this stage, the person with Alzheimer's is usually capable of communicating basic ideas adequately.[41][44][45] While performing fine motor tasks such as writing, drawing, or dressing, certain movement coordination and planning difficulties (apraxia) may be present; however, they are commonly unnoticed.[41] As the disease progresses, people with Alzheimer's disease can often continue to perform many tasks independently; however, they may need assistance or supervision with the most cognitively demanding activities.[41]
Middle stage
Progressive deterioration eventually hinders independence, with subjects being unable to perform most common activities of daily living.[41] Speech difficulties become evident due to an inability to recall vocabulary, which leads to frequent incorrect word substitutions (paraphasias). Reading and writing skills are also progressively lost.[41][45] Complex motor sequences become less coordinated as time passes and Alzheimer's disease progresses, so the risk of falling increases.[41] During this phase, memory problems worsen, and the person may fail to recognise close relatives.[41] Long-term memory, which was previously intact, becomes impaired.[41]
Behavioral and neuropsychiatric changes become more prevalent. Common manifestations are wandering, irritability and emotional lability, leading to crying, outbursts of unpremeditated aggression, or resistance to caregiving.[41] Sundowning can also appear.[46] Approximately 30% of people with Alzheimer's disease develop illusionary misidentifications and other delusional symptoms.[41] Subjects also lose insight of their disease process and limitations (anosognosia).[41] Urinary incontinence can develop.[41] These symptoms create stress for relatives and caregivers, which can be reduced by moving the person from home care to other long-term care facilities.[41][47]
Late stage

During the final stage, known as the late-stage or severe stage, there is complete dependence on caregivers.[19][33][41] Language is reduced to simple phrases or even single words, eventually leading to complete loss of speech.[41][45] Despite the loss of verbal language abilities, people can often understand and return emotional signals. Although aggressiveness can still be present, extreme apathy and exhaustion are much more common symptoms. People with Alzheimer's disease will ultimately not be able to perform even the simplest tasks independently; muscle mass and mobility deteriorates to the point where they are bedridden and unable to feed themselves. The cause of death is usually an external factor, such as infection of pressure ulcers or pneumonia, not the disease itself.[41] In some cases, there is a paradoxical lucidity immediately before death, where there is an unexpected recovery of mental clarity.[48]
Causes
Alzheimer's disease is believed to occur when abnormal amounts of amyloid beta (Aβ), accumulating extracellularly as amyloid plaques and tau proteins, or intracellularly as neurofibrillary tangles, form in the brain, affecting neuronal functioning and connectivity, resulting in a progressive loss of brain function.[49][50] This altered protein clearance ability is age-related, regulated by brain cholesterol,[51] and associated with other neurodegenerative diseases.[52][53]
The cause for most Alzheimer's cases is still mostly unknown,[14] except for 1–2% of cases where deterministic genetic differences have been identified.[17] Several competing hypotheses attempt to explain the underlying cause; the most predominant hypothesis is the amyloid beta (Aβ) hypothesis.[14]
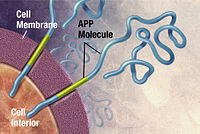
The oldest hypothesis, on which most drug therapies are based, is the cholinergic hypothesis, which proposes that Alzheimer's disease is caused by reduced synthesis of the neurotransmitter acetylcholine.[14] The loss of cholinergic neurons noted in the limbic system and cerebral cortex, is a key feature in the progression of Alzheimer's.[39] The 1991 amyloid hypothesis postulated that extracellular amyloid beta (Aβ) deposits are the fundamental cause of the disease.[54][55] Support for this postulate comes from the location of the gene for the amyloid precursor protein (APP) on chromosome 21, together with the fact that people with trisomy 21 (Down syndrome) who have an extra gene copy almost universally exhibit at least the earliest symptoms of Alzheimer's disease by 40 years of age.[10] A specific isoform of apolipoprotein, APOE4, is a major genetic risk factor for Alzheimer's disease.[15] While apolipoproteins enhance the breakdown of beta amyloid, some isoforms are not very effective at this task (such as APOE4), leading to excess amyloid buildup in the brain.[56]
Genetic
Late onset
Late-onset Alzheimer's is about 70% heritable.[57][58] Genetic models in 2020 predict Alzheimer's disease with 90% accuracy.[59] Most cases of Alzheimer's are not familial, and so they are termed sporadic Alzheimer's disease.[60] Of the cases of sporadic Alzheimer's disease, most are classified as late onset where they are developed after the age of 65 years.[61]
The strongest genetic risk factor for sporadic Alzheimer's disease is APOEε4.[18] APOEε4 is one of four alleles of apolipoprotein E (APOE). APOE plays a major role in lipid-binding proteins in lipoprotein particles and the ε4 allele disrupts this function.[62] Between 40% and 80% of people with Alzheimer's disease possess at least one APOEε4 allele.[63] The APOEε4 allele increases the risk of the disease by three times in heterozygotes and by 15 times in homozygotes.[64] Like many human diseases, environmental effects and genetic modifiers result in incomplete penetrance. For example, Nigerian Yoruba people do not show the relationship between dose of APOEε4 and incidence or age-of-onset for Alzheimer's disease seen in other human populations.[65][66]
Early onset
Only 1–2% of Alzheimer's cases are inherited due to autosomal dominant effects, as Alzheimer's is highly polygenic. When the disease is caused by autosomal dominant variants, it is known as early onset familial Alzheimer's disease, which is rarer and has a faster rate of progression.[17] Less than 5% of sporadic Alzheimer's disease have an earlier onset,[17] and early-onset Alzheimer's is about 90% heritable.[57][58] Familial Alzheimer's disease usually implies two or more persons affected in one or more generations.[67][68][69]
Early onset familial Alzheimer's disease can be attributed to mutations in one of three genes: those encoding amyloid-beta precursor protein (APP) and presenilins PSEN1 and PSEN2.[40] Most mutations in the APP and presenilin genes increase the production of a small protein called amyloid beta (Aβ)42, which is the main component of amyloid plaques.[70] Some of the mutations merely alter the ratio between Aβ42 and the other major forms—particularly Aβ40—without increasing Aβ42 levels in the brain.[71] Two other genes associated with autosomal dominant Alzheimer's disease are ABCA7 and SORL1.[72]
Alleles in the TREM2 gene have been associated with a three to five times higher risk of developing Alzheimer's disease.[73]
A Japanese pedigree of familial Alzheimer's disease was found to be associated with a deletion mutation of codon 693 of APP.[74] This mutation and its association with Alzheimer's disease was first reported in 2008,[75] and is known as the Osaka mutation. Only homozygotes with this mutation have an increased risk of developing Alzheimer's disease. This mutation accelerates Aβ oligomerization but the proteins do not form the amyloid fibrils that aggregate into amyloid plaques, suggesting that it is the Aβ oligomerization rather than the fibrils that may be the cause of this disease. Mice expressing this mutation have all the usual pathologies of Alzheimer's disease.[76]
Hypotheses
Amyloid beta and tau protein

The tau hypothesis proposes that tau protein abnormalities initiate the disease cascade.[77] In this model, hyperphosphorylated tau begins to pair with other threads of tau as paired helical filaments. Eventually, they form neurofibrillary tangles inside nerve cell bodies.[77] When this occurs, the microtubules disintegrate, destroying the structure of the cell's cytoskeleton which collapses the neuron's transport system.[77]
A number of studies connect the misfolded amyloid beta and tau proteins associated with the pathology of Alzheimer's disease, as bringing about oxidative stress that leads to neuroinflammation.[78] This chronic inflammation is also a feature of other neurodegenerative diseases including Parkinson's disease, and ALS.[79] Spirochete infections have also been linked to dementia.[14] DNA damages accumulate in Alzheimer's diseased brains; reactive oxygen species may be the major source of this DNA damage.[80]
Sleep
Sleep disturbances are seen as a possible risk factor for inflammation in Alzheimer's disease.[81] Sleep disruption was previously only seen as a consequence of Alzheimer's disease, but as of 2020[update], accumulating evidence suggests that this relationship may be bidirectional.[82]
Metal toxicity, smoking, neuroinflammation and air pollution
The cellular homeostasis of biometals such as ionic copper, iron, and zinc is disrupted in Alzheimer's disease, though it remains unclear whether this is produced by or causes the changes in proteins.[14][83] Smoking is a significant Alzheimer's disease risk factor.[1] Systemic markers of the innate immune system are risk factors for late-onset Alzheimer's disease.[84] Exposure to air pollution may be a contributing factor to the development of Alzheimer's disease.[14]
Age-related myelin decline
Retrogenesis is a medical hypothesis that just as the fetus goes through a process of neurodevelopment beginning with neurulation and ending with myelination, the brains of people with Alzheimer's disease go through a reverse neurodegeneration process starting with demyelination and death of axons (white matter) and ending with the death of grey matter.[85] Likewise the hypothesis is, that as infants go through states of cognitive development, people with Alzheimer's disease go through the reverse process of progressive cognitive impairment.[86]
According to one theory, dysfunction of oligodendrocytes and their associated myelin during aging contributes to axon damage, which in turn generates in amyloid production and tau hyperphosphorylation.[87][88] An in vivo study employing genetic mouse models to simulate myelin dysfunction and amyloidosis further reveal that age-related myelin degradation increases sites of Aβ production and distracts microglia from Aβ plaques, with both mechanisms dually exacerbating amyloidosis.[89] Additionally, comorbidities between the demyelinating disease, multiple sclerosis, and Alzheimer's disease have been reported.[90][91]
Other hypotheses
The association with celiac disease is unclear, with a 2019 study finding no increase in dementia overall in those with celiac disease while a 2018 review found an association with several types of dementia including Alzheimer's disease.[92][93]
Studies have shown a potential link between infection with certain viruses and developing Alzheimer's disease later in life.[94] Notably, a large scale study conducted on 6,245,282 patients has shown an increased risk of developing Alzheimer's disease following COVID-19 infection in cognitively normal individuals over 65.[95]
Pathophysiology

Neuropathology
Alzheimer's disease is characterised by loss of neurons and synapses in the cerebral cortex and certain subcortical regions. This loss results in gross atrophy of the affected regions, including degeneration in the temporal lobe and parietal lobe, and parts of the frontal cortex and cingulate gyrus.[96] Degeneration is also present in brainstem nuclei particularly the locus coeruleus in the pons.[97] Studies using MRI and PET have documented reductions in the size of specific brain regions in people with Alzheimer's disease as they progressed from mild cognitive impairment to Alzheimer's disease, and in comparison with similar images from healthy older adults.[98][99]
Both Aβ plaques and neurofibrillary tangles are clearly visible by microscopy in brains of those with Alzheimer's disease,[100] especially in the hippocampus.[101] However, Alzheimer's disease may occur without neurofibrillary tangles in the neocortex.[102] Plaques are dense, mostly insoluble deposits of beta-amyloid peptide and cellular material outside and around neurons. Neurofibrillary tangles are aggregates of the microtubule-associated protein tau which has become hyperphosphorylated and accumulate inside the cells themselves. Although many older individuals develop some plaques and tangles as a consequence of aging, the brains of people with Alzheimer's disease have a greater number of them in specific brain regions such as the temporal lobe.[103] Lewy bodies are not rare in the brains of people with Alzheimer's disease.[104]
Biochemistry
Amyloid beta
Alzheimer's disease has been identified as a protein misfolding disease, a proteopathy, caused by the accumulation of abnormally folded amyloid beta protein into amyloid plaques, and tau protein into neurofibrillary tangles in the brain.[77] Plaques are made up of small peptides, 39–43 amino acids in length, called amyloid beta. Amyloid beta is a fragment from the larger amyloid-beta precursor protein (APP) a transmembrane protein that penetrates the cell's membrane. APP is critical to neuron growth, survival, and post-injury repair.[77] In Alzheimer's disease, gamma secretase and beta secretase act together in a proteolytic process which causes APP to be divided into smaller fragments.[77] Although commonly researched as neuronal proteins, APP and its processing enzymes are abundantly expressed by other brain cells. One of these fragments gives rise to fibrils of amyloid beta, which then form clumps that deposit outside neurons in dense formations known as amyloid plaques.[77] Excitatory neurons are known to be the major producers of amyloid beta that contribute to major extracellular plaque deposition.[77]
Phosphorylated tau
Alzheimer's disease is also considered a tauopathy due to abnormal aggregation of the tau protein. Every neuron has a cytoskeleton, an internal support structure partly made up of structures called microtubules. These microtubules act like tracks, guiding nutrients and molecules from the body of the cell to the ends of the axon and back. A protein called tau stabilises the microtubules when phosphorylated, and is therefore called a microtubule-associated protein. In Alzheimer's disease, tau undergoes chemical changes, becoming hyperphosphorylated; it then begins to pair with other threads, creating neurofibrillary tangles and disintegrating the neuron's transport system.[105] Pathogenic tau can also cause neuronal death through transposable element dysregulation.[106] Necroptosis has also been reported as a mechanism of cell death in brain cells affected with tau tangles.[107][108]
Disease mechanism
Exactly how disturbances of production and aggregation of the beta-amyloid peptide give rise to the pathology of Alzheimer's disease is not known.[109][110] The amyloid hypothesis traditionally points to the accumulation of beta-amyloid peptides as the central event triggering neuron degeneration. Accumulation of aggregated amyloid fibrils, which are believed to be the toxic form of the protein responsible for disrupting the cell's calcium ion homeostasis, induces programmed cell death (apoptosis).[111] It is also known that Aβ selectively builds up in the mitochondria in the cells of Alzheimer's-affected brains, and it also inhibits certain enzyme functions and the utilisation of glucose by neurons.[112]
Iron dyshomeostasis is linked to disease progression, an iron-dependent form of regulated cell death called ferroptosis could be involved. Products of lipid peroxidation are also elevated in AD brain compared with controls.[113]
Various inflammatory processes and cytokines may also have a role in the pathology of Alzheimer's disease. Inflammation is a general marker of tissue damage in any disease, and may be either secondary to tissue damage in Alzheimer's disease or a marker of an immunological response.[114] There is increasing evidence of a strong interaction between the neurons and the immunological mechanisms in the brain. Obesity and systemic inflammation may interfere with immunological processes which promote disease progression.[115]
Alterations in the distribution of different neurotrophic factors and in the expression of their receptors such as the brain-derived neurotrophic factor (BDNF) have been described in Alzheimer's disease.[116][117]
Diagnosis

Alzheimer's disease (AD) can only be definitively diagnosed with autopsy findings; in the absence of autopsy, clinical diagnoses of AD are "possible" or "probable", based on other findings.[21][22][118] Up to 23% of those clinically diagnosed with AD may be misdiagnosed and may have pathology suggestive of another condition with symptoms that mimic those of AD.[22]
AD is usually clinically diagnosed based on a person's medical history, observations from friends or relatives, and behavioral changes. The presence of characteristic neuropsychological changes with impairments in at least two cognitive domains that are severe enough to affect a person's functional abilities are required for the diagnosis. Domains that may be impaired include memory (most commonly impaired), language, executive function, visuospatial functioning, or other areas of cognition. The neurocognitive changes must be a decline from a prior level of function and the diagnosis requires ruling out other common causes of neurocognitive decline.[119][120][121] Advanced medical imaging with computed tomography (CT) or magnetic resonance imaging (MRI), and with single-photon emission computed tomography (SPECT) or positron emission tomography (PET), can be used to help exclude other cerebral pathology or subtypes of dementia.[122] On MRI or CT, Alzheimer's disease usually shows a generalized or focal cortical atrophy, which may be asymmetric. Atrophy of the hippocampus is also commonly seen. Brain imaging commonly also shows cerebrovascular disease, most commonly previous strokes (small or large territory strokes), and this is thought to be a contributing cause of many cases of dementia (up to 46% cases of dementia also have cerebrovascular disease on imaging).[119] FDG-PET scan is not required for the diagnosis but it is sometimes used when standard testing is unclear. FDG-PET shows a bilateral, asymetric, temporal and parietal reduced activity.[119] Advanced imaging may predict conversion from prodromal stages (mild cognitive impairment) to Alzheimer's disease.[123] FDA-approved radiopharmaceutical diagnostic agents used in PET for Alzheimer's disease are florbetapir (2012), flutemetamol (2013), florbetaben (2014), and flortaucipir (2020).[124] Because many insurance companies in the United States do not cover this procedure, its use in clinical practice is largely limited to clinical trials as of 2018[update].[125]
Assessment of intellectual functioning including memory testing can further characterise the state of the disease.[1] Medical organizations have created diagnostic criteria to ease and standardise the diagnostic process for practising physicians. Definitive diagnosis can only be confirmed with post-mortem evaluations when brain material is available and can be examined histologically for senile plaques and neurofibrillary tangles.[125][126]
Criteria
There are three sets of criteria for the clinical diagnoses of the spectrum of Alzheimer's disease: the 2013 fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5); the National Institute on Aging-Alzheimer's Association (NIA-AA) definition as revised in 2011; and the International Working Group criteria as revised in 2010.[40][125] Three broad time periods, which can span decades, define the progression of Alzheimer's disease from the preclinical phase, to mild cognitive impairment (MCI), followed by Alzheimer's disease dementia.[127]
Eight intellectual domains are most commonly impaired in AD—memory, language, perceptual skills, attention, motor skills, orientation, problem solving and executive functional abilities, as listed in the fourth text revision of the DSM (DSM-IV-TR).[128]
The DSM-5 defines criteria for probable or possible AD for both major and mild neurocognitive disorder.[129][130][118] Major or mild neurocognitive disorder must be present along with at least one cognitive deficit for a diagnosis of either probable or possible AD.[129][131] For major neurocognitive disorder due to AD, probable Alzheimer's disease can be diagnosed if the individual has genetic evidence of AD[132] or if two or more acquired cognitive deficits, and a functional disability that is not from another disorder, are present.[133] Otherwise, possible AD can be diagnosed as the diagnosis follows an atypical route.[134] For mild neurocognitive disorder due to AD, probable Alzheimer's disease can be diagnosed if there is genetic evidence, whereas possible AD can be met if all of the following are present: no genetic evidence, decline in both learning and memory, two or more cognitive deficits, and a functional disability not from another disorder.[129][135]
The NIA-AA criteria are used mainly in research rather than in clinical assessments.[136] They define AD through three major stages: preclinical, mild cognitive impairment (MCI), and Alzheimer's dementia.[137][138] Diagnosis in the preclinical stage is complex and focuses on asymptomatic individuals;[138][139] the latter two stages describe individuals experiencing symptoms.[138] The core clinical criteria for MCI is used along with identification of biomarkers,[140] predominantly those for neuronal injury (mainly tau-related) and amyloid beta deposition.[136][138] The core clinical criteria itself rests on the presence of cognitive impairment[138] without the presence of comorbidities.[141][142] The third stage is divided into probable and possible AD dementia.[142] In probable AD dementia there is steady impairment of cognition over time and a memory-related or non-memory-related cognitive dysfunction.[142] In possible AD dementia, another causal disease such as cerebrovascular disease is present.[142]
Techniques

Neuropsychological tests including cognitive tests such as the mini–mental state examination (MMSE), the Montreal Cognitive Assessment (MoCA) and the Mini-Cog are widely used to aid in diagnosis of the cognitive impairments in AD.[143] These tests may not always be accurate, as they lack sensitivity to mild cognitive impairment, and can be biased by language or attention problems;[143] more comprehensive test arrays are necessary for high reliability of results, particularly in the earliest stages of the disease.[144][145]
Further neurological examinations are crucial in the differential diagnosis of Alzheimer's disease and other diseases.[34] Interviews with family members are used in assessment; caregivers can supply important information on daily living abilities and on the decrease in the person's mental function.[146] A caregiver's viewpoint is particularly important, since a person with Alzheimer's disease is commonly unaware of their deficits.[147] Many times, families have difficulties in the detection of initial dementia symptoms and may not communicate accurate information to a physician.[148]
Supplemental testing can rule out other potentially treatable diagnoses and help avoid misdiagnoses.[149] Common supplemental tests include blood tests, thyroid function tests, as well as tests to assess vitamin B12 levels, rule out neurosyphilis and rule out metabolic problems (including tests for kidney function, electrolyte levels and for diabetes).[149] MRI or CT scans might also be used to rule out other potential causes of the symptoms – including tumors or strokes.[143] Delirium and depression can be common among individuals and are important to rule out.[150]
Psychological tests for depression are used, since depression can either be concurrent with AD (see Depression of Alzheimer disease), an early sign of cognitive impairment,[151] or even the cause.[152][153]
Due to low accuracy, the C-PIB-PET scan is not recommended as an early diagnostic tool or for predicting the development of AD when people show signs of mild cognitive impairment (MCI).[154] The use of 18F-FDG PET scans, as a single test, to identify people who may develop Alzheimer's disease is not supported by evidence.[155]
Prevention

There are no disease-modifying treatments available to cure Alzheimer's disease and because of this, AD research has focused on interventions to prevent the onset and progression.[156] There is no evidence that supports any particular measure in preventing AD,[1] and studies of measures to prevent the onset or progression have produced inconsistent results. Epidemiological studies have proposed relationships between an individual's likelihood of developing AD and modifiable factors, such as medications, lifestyle, and diet. There are some challenges in determining whether interventions for AD act as a primary prevention method, preventing the disease itself, or a secondary prevention method, identifying the early stages of the disease.[157] These challenges include duration of intervention, different stages of disease at which intervention begins, and lack of standardization of inclusion criteria regarding biomarkers specific for AD.[157] Further research is needed to determine factors that can help prevent AD.[157]
Medication
Cardiovascular risk factors, such as hypercholesterolaemia, hypertension, diabetes, and smoking, are associated with a higher risk of onset and worsened course of AD.[158][159] The use of statins to lower cholesterol may be of benefit in AD.[160] Antihypertensive and antidiabetic medications in individuals without overt cognitive impairment may decrease the risk of dementia by influencing cerebrovascular pathology.[1][161] More research is needed to examine the relationship with AD specifically; clarification of the direct role medications play versus other concurrent lifestyle changes (diet, exercise, smoking) is needed.[1]
Depression is associated with an increased risk for AD; management with antidepressant medications may provide a preventative measure.[5]
Historically, long-term usage of non-steroidal anti-inflammatory drugs (NSAIDs) were thought to be associated with a reduced likelihood of developing AD as it reduces inflammation, but NSAIDs do not appear to be useful as a treatment.[125] Additionally, because women have a higher incidence of AD than men, it was once thought that estrogen deficiency during menopause was a risk factor, but there is a lack of evidence to show that hormone replacement therapy (HRT) in menopause decreases risk of cognitive decline.[162]
Lifestyle
Certain lifestyle activities, such as physical and cognitive exercises, higher education and occupational attainment, cigarette smoking, stress, sleep, and the management of other comorbidities, including diabetes and hypertension, may affect the risk of developing AD.[5]
Physical exercise is associated with a decreased rate of dementia,[6] and is effective in reducing symptom severity in those with AD.[163] Memory and cognitive functions can be improved with aerobic exercises including brisk walking three times weekly for forty minutes.[164] It may also induce neuroplasticity of the brain.[165] Participating in mental exercises, such as reading, crossword puzzles, and chess have shown potential to be preventive.[5] Meeting the WHO recommendations for physical activity is associated with a lower risk of AD.[166]
Higher education and occupational attainment, and participation in leisure activities, contribute to a reduced risk of developing AD,[7] or of delaying the onset of symptoms. This is compatible with the cognitive reserve theory, which states that some life experiences result in more efficient neural functioning providing the individual a cognitive reserve that delays the onset of dementia manifestations.[7] Education delays the onset of Alzheimer's disease syndrome without changing the duration of the disease.[167]
Cessation in smoking may reduce risk of developing AD, specifically in those who carry the APOE ɛ4 allele.[168][5] The increased oxidative stress caused by smoking results in downstream inflammatory or neurodegenerative processes that may increase risk of developing AD.[169] Avoidance of smoking, counseling and pharmacotherapies to quit smoking are used, and avoidance of environmental tobacco smoke is recommended.[5]
Alzheimer's disease is associated with sleep disorders but the precise relationship is unclear.[170][171] It was once thought that as people get older, the risk of developing sleep disorders and AD independently increase, but research is examining whether sleep disorders may increase the prevalence of AD.[170] One theory is that the mechanisms to increase clearance of toxic substances, including Aβ, are active during sleep.[170][172] With decreased sleep, a person is increasing Aβ production and decreasing Aβ clearance, resulting in Aβ accumulation.[173][170][171] Receiving adequate sleep (approximately 7–8 hours) every night has become a potential lifestyle intervention to prevent the development of AD.[5]
Stress is a risk factor for the development of AD.[5] The mechanism by which stress predisposes someone to development of AD is unclear, but it is suggested that lifetime stressors may affect a person's epigenome, leading to an overexpression or under expression of specific genes.[174] Although the relationship of stress and AD is unclear, strategies to reduce stress and relax the mind may be helpful strategies in preventing the progression or Alzheimer's disease.[175] Meditation, for instance, is a helpful lifestyle change to support cognition and well-being, though further research is needed to assess long-term effects.[165]
Management
There is no cure for AD;[176] available treatments offer relatively small symptomatic benefits but remain palliative in nature.[14][177] Treatments can be divided into pharmaceutical, psychosocial, and caregiving.
Pharmaceutical


Medications used to treat the cognitive symptoms of AD rather than the underlying cause include: four acetylcholinesterase inhibitors (tacrine, rivastigmine, galantamine, and donepezil) and memantine, an NMDA receptor antagonist. The acetylcholinesterase inhibitors are intended for those with mild to severe AD, whereas memantine is intended for those with moderate or severe Alzheimer's disease.[125] The benefit from their use is small.[178][179][180][15]
Reduction in the activity of the cholinergic neurons is a well-known feature of AD.[181] Acetylcholinesterase inhibitors are employed to reduce the rate at which acetylcholine (ACh) is broken down, thereby increasing the concentration of ACh in the brain and combating the loss of ACh caused by the death of cholinergic neurons.[182] There is evidence for the efficacy of these medications in mild to moderate AD,[183][178] and some evidence for their use in the advanced stage.[178] The use of these drugs in mild cognitive impairment has not shown any effect in a delay of the onset of Alzheimer's disease.[184] The most common side effects are nausea and vomiting, both of which are linked to cholinergic excess. These side effects arise in approximately 10–20% of users, are mild to moderate in severity, and can be managed by slowly adjusting medication doses.[185] Less common secondary effects include muscle cramps, decreased heart rate (bradycardia), decreased appetite and weight, and increased gastric acid production.[183]
Glutamate is an excitatory neurotransmitter of the nervous system, although excessive amounts in the brain can lead to cell death through a process called excitotoxicity which consists of the overstimulation of glutamate receptors. Excitotoxicity occurs not only in AD, but also in other neurological diseases such as Parkinson's disease and multiple sclerosis.[186] Memantine is a noncompetitive NMDA receptor antagonist first used as an anti-influenza agent. It acts on the glutamatergic system by blocking NMDA receptors and inhibiting their overstimulation by glutamate.[186][187] Memantine has been shown to have a small benefit in the treatment of moderate to severe AD.[188] Reported adverse events with memantine are infrequent and mild, including hallucinations, confusion, dizziness, headache and fatigue.[189][190] The combination of memantine and donepezil[191] has been shown to be "of statistically significant but clinically marginal effectiveness".[192]
An extract of Ginkgo biloba known as EGb 761 has been used for treating AD and other neuropsychiatric disorders.[193] Its use is approved throughout Europe.[194] The World Federation of Biological Psychiatry guidelines lists EGb 761 with the same weight of evidence (level B) given to acetylcholinesterase inhibitors and memantine. EGb 761 is the only one that showed improvement of symptoms in both AD and vascular dementia. EGb 761 may have a role either on its own or as an add-on if other therapies prove ineffective.[193] A 2016 review concluded that the quality of evidence from clinical trials on Ginkgo biloba has been insufficient to warrant its use for treating AD.[195]
Atypical antipsychotics are modestly useful in reducing aggression and psychosis in people with AD, but their advantages are offset by serious adverse effects, such as stroke, movement difficulties or cognitive decline.[196] When used in the long-term, they have been shown to associate with increased mortality.[197] They are recommended in dementia only after first line therapies such as behavior modification have failed, and due to the risk of adverse effects, they should be used for the shortest amount of time possible.[119] Stopping antipsychotic use in this group of people appears to be safe.[198]
Psychosocial
Psychosocial interventions are used as an adjunct to pharmaceutical treatment and can be classified within behavior-, emotion-, cognition- or stimulation-oriented approaches.[needs update][199]
Behavioral interventions attempt to identify and reduce the antecedents and consequences of problem behaviors. This approach has not shown success in improving overall functioning,[200] but can help to reduce some specific problem behaviors, such as incontinence.[201] There is a lack of high quality data on the effectiveness of these techniques in other behavior problems such as wandering.[202][203] Music therapy is effective in reducing behavioral and psychological symptoms.[204]
Emotion-oriented interventions include reminiscence therapy, validation therapy, supportive psychotherapy, sensory integration, also called snoezelen, and simulated presence therapy. A Cochrane review has found no evidence that this is effective.[205] Reminiscence therapy (RT) involves the discussion of past experiences individually or in group, many times with the aid of photographs, household items, music and sound recordings, or other familiar items from the past. A 2018 review of the effectiveness of RT found that effects were inconsistent, small in size and of doubtful clinical significance, and varied by setting.[206] Simulated presence therapy (SPT) is based on attachment theories and involves playing a recording with voices of the closest relatives of the person with AD. There is partial evidence indicating that SPT may reduce challenging behaviors.[207]
The aim of cognition-oriented treatments, which include reality orientation and cognitive retraining, is the reduction of cognitive deficits. Reality orientation consists of the presentation of information about time, place, or person to ease the understanding of the person about its surroundings and his or her place in them. On the other hand, cognitive retraining tries to improve impaired capacities by exercising mental abilities. Both have shown some efficacy improving cognitive capacities.[208]
Stimulation-oriented treatments include art, music and pet therapies, exercise, and any other kind of recreational activities. Stimulation has modest support for improving behavior, mood, and, to a lesser extent, function. Nevertheless, as important as these effects are, the main support for the use of stimulation therapies is the change in the person's routine.[199]
Caregiving
Since AD has no cure and it gradually renders people incapable of tending to their own needs, caregiving is essentially the treatment and must be carefully managed over the course of the disease.
During the early and moderate stages, modifications to the living environment and lifestyle can increase safety and reduce caretaker burden.[209][210] Examples of such modifications are the adherence to simplified routines, the placing of safety locks, the labeling of household items to cue the person with the disease or the use of modified daily life objects.[199][211][212] If eating becomes problematic, food will need to be prepared in smaller pieces or even puréed.[213] When swallowing difficulties arise, the use of feeding tubes may be required. In such cases, the medical efficacy and ethics of continuing feeding is an important consideration of the caregivers and family members.[214][215] The use of physical restraints is rarely indicated in any stage of the disease, although there are situations when they are necessary to prevent harm to the person with Alzheimer's disease or their caregivers.[199]
During the final stages of the disease, treatment is centred on relieving discomfort until death, often with the help of hospice.[216]
Diet
Diet may be a modifiable risk factor for the development of Alzheimer's disease. The Mediterranean diet, and the DASH diet are both associated with less cognitive decline. A different approach has been to incorporate elements of both of these diets into one known as the MIND diet.[217] Studies of individual dietary components, minerals and supplements are conflicting as to whether they prevent AD or cognitive decline.[217]
Prognosis
The early stages of AD are difficult to diagnose. A definitive diagnosis is usually made once cognitive impairment compromises daily living activities, although the person may still be living independently. The symptoms will progress from mild cognitive problems, such as memory loss through increasing stages of cognitive and non-cognitive disturbances, eliminating any possibility of independent living, especially in the late stages of the disease.[41]
Life expectancy of people with AD is reduced.[218] The normal life expectancy for 60 to 70 years old is 23 to 15 years; for 90 years old it is 4.5 years.[219] Following AD diagnosis it ranges from 7 to 10 years for those in their 60s and early 70s (a loss of 13 to 8 years), to only about 3 years or less (a loss of 1.5 years) for those in their 90s.[218]
Fewer than 3% of people live more than fourteen years after diagnosis.[220] Disease features significantly associated with reduced survival are an increased severity of cognitive impairment, decreased functional level, disturbances in the neurological examination, history of falls, malnutrition, dehydration and weight loss.[3] Other coincident diseases such as heart problems, diabetes, or history of alcohol abuse are also related with shortened survival.[221][222][223] While the earlier the age at onset the higher the total survival years, life expectancy is particularly reduced when compared to the healthy population among those who are younger.[224] Men have a less favourable survival prognosis than women.[needs update][220][225]
Aspiration pneumonia is the most frequent immediate cause of death brought by AD.[3] While the reasons behind the lower prevalence of cancer in AD patients remain unclear, some researchers hypothesize that biological mechanisms shared by both diseases might play a role. However, this requires further investigation.[226]
Epidemiology
Two main measures are used in epidemiological studies: incidence and prevalence. Incidence is the number of new cases per unit of person-time at risk (usually number of new cases per thousand person-years); while prevalence is the total number of cases of the disease in the population at any given time.

Regarding incidence, cohort longitudinal studies (studies where a disease-free population is followed over the years) provide rates between 10 and 15 per thousand person-years for all dementias and 5–8 for AD,[227][228] which means that half of new dementia cases each year are Alzheimer's disease. Advancing age is a primary risk factor for the disease and incidence rates are not equal for all ages: every 5 years after the age of 65, the risk of acquiring the disease approximately doubles, increasing from 3 to as much as 69 per thousand person years.[227][228] Females with AD are more common than males, but this difference is likely due to women's longer life spans. When adjusted for age, both sexes are affected by Alzheimer's at equal rates.[15] In the United States, the risk of dying from AD in 2010 was 26% higher among the non-Hispanic white population than among the non-Hispanic black population, and the Hispanic population had a 30% lower risk than the non-Hispanic white population.[229] However, much AD research remains to be done in minority groups, such as the African American, East Asian and Hispanic/Latino populations.[230][231] Studies have shown that these groups are underrepresented in clinical trials and do not have the same risk of developing AD when carrying certain genetic risk factors (i.e. APOE4), compared to their caucasian counterparts.[231][232][233]
The prevalence of AD in populations is dependent upon factors including incidence and survival. Since the incidence of AD increases with age, prevalence depends on the mean age of the population for which prevalence is given. In the United States in 2020, AD dementia prevalence was estimated to be 5.3% for those in the 60–74 age group, with the rate increasing to 13.8% in the 74–84 group and to 34.6% in those greater than 85.[234] Prevalence rates in some less developed regions around the globe are lower.[235][236] Both the prevalence and incidence rates of AD are steadily increasing, however the prevalence rate is estimated to triple by 2050 reaching 152 million, compared to the 50 million people with AD globally in 2020.[14][237][238]
History

The ancient Greek and Roman philosophers and physicians associated old age with increasing dementia.[29] It was not until 1901 that German psychiatrist Alois Alzheimer identified the first case of what became known as Alzheimer's disease, named after him, in a fifty-year-old woman he called Auguste D. He followed her case until she died in 1906 when he first reported publicly on it.[239] During the next five years, eleven similar cases were reported in the medical literature, some of them already using the term Alzheimer's disease.[29] The disease was first described as a distinctive disease by Emil Kraepelin after suppressing some of the clinical (delusions and hallucinations) and pathological features (arteriosclerotic changes) contained in the original report of Auguste D.[240] He included Alzheimer's disease, also named presenile dementia by Kraepelin, as a subtype of senile dementia in the eighth edition of his Textbook of Psychiatry, published on 15 July 1910.[241]
For most of the 20th century, the diagnosis of Alzheimer's disease was reserved for individuals between the ages of 45 and 65 who developed symptoms of dementia. The terminology changed after 1977 when a conference on Alzheimer's disease concluded that the clinical and pathological manifestations of presenile and senile dementia were almost identical, although the authors also added that this did not rule out the possibility that they had different causes.[242] This eventually led to the diagnosis of Alzheimer's disease independent of age.[243] The term senile dementia of the Alzheimer type (SDAT) was used for a time to describe the condition in those over 65, with classical Alzheimer's disease being used to describe those who were younger. Eventually, the term Alzheimer's disease was formally adopted in medical nomenclature to describe individuals of all ages with a characteristic common symptom pattern, disease course, and neuropathology.[244]
The National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer's Disease and Related Disorders Association (ADRDA, now known as the Alzheimer's Association) established the most commonly used NINCDS-ADRDA Alzheimer's Criteria for diagnosis in 1984,[245] extensively updated in 2007.[246][149] These criteria require that the presence of cognitive impairment, and a suspected dementia syndrome, be confirmed by neuropsychological testing for a clinical diagnosis of possible or probable Alzheimer's disease. A histopathologic confirmation including a microscopic examination of brain tissue is required for a definitive diagnosis. Good statistical reliability and validity have been shown between the diagnostic criteria and definitive histopathological confirmation.[247]
Society and culture
Social costs
Dementia, and specifically Alzheimer's disease, may be among the most costly diseases for societies worldwide.[248] As populations age, these costs will probably increase and become an important social problem and economic burden.[249] Costs associated with AD include direct and indirect medical costs, which vary between countries depending on social care for a person with AD.[248][250][251] Direct costs include doctor visits, hospital care, medical treatments, nursing home care, specialized equipment, and household expenses.[248][249] Indirect costs include the cost of informal care and the loss in productivity of informal caregivers.[249]
In the United States as of 2019[update], informal (family) care is estimated to constitute nearly three-fourths of caregiving for people with AD at a cost of US$234 billion per year and approximately 18.5 billion hours of care.[248] The cost to society worldwide to care for individuals with AD is projected to increase nearly ten-fold, and reach about US$9.1 trillion by 2050.[250]
Costs for those with more severe dementia or behavioral disturbances are higher and are related to the additional caregiving time to provide physical care.[251]
Caregiving burden
This section needs to be updated. (February 2022) |
Individuals with Alzheimer's will require assistance in their lifetime, and care will most likely come in the form of a full-time caregiver which is often a role that is taken on by the spouse or a close relative. Caregiving tends to include physical and emotional burdens as well as time and financial strain at times on the person administering the aid.[252][253] Alzheimer's disease is known for placing a great burden on caregivers which includes social, psychological, physical, or economic aspects.[23][254][255] Home care is usually preferred by both those people with Alzheimer's disease as well as their families.[256] This option also delays or eliminates the need for more professional and costly levels of care.[256][257] Nevertheless, two-thirds of nursing home residents have dementias.[199]
Dementia caregivers are subject to high rates of physical and mental disorders.[258] Factors associated with greater psychosocial problems of the primary caregivers include having an affected person at home, the caregiver being a spouse, demanding behaviors of the cared person such as depression, behavioral disturbances, hallucinations, sleep problems or walking disruptions and social isolation.[259][260] In the United States, the yearly cost of caring for a person with dementia ranges from $41,689-$56,290 per year.[261] Other estimates range from $28,078-$56,022 per year for formal medical care and $36,667-$92,689 for informal care provided by a relative or friend (assuming market value replacement costs for the care provided by the informal caregiver) and $15,792-$71,813 in lost wages.[262]
Cognitive behavioral therapy and the teaching of coping strategies either individually or in group have demonstrated their efficacy in improving caregivers' psychological health.[23][263]
Media
Alzheimer's disease has been portrayed in films such as: Iris (2001), based on John Bayley's memoir of his wife Iris Murdoch;[264] The Notebook (2004), based on Nicholas Sparks's 1996 novel of the same name;[265] A Moment to Remember (2004); Thanmathra (2005);[266] Memories of Tomorrow (Ashita no Kioku) (2006), based on Hiroshi Ogiwara's novel of the same name;[267] Away from Her (2006), based on Alice Munro's short story The Bear Came over the Mountain;[268] Still Alice (2014), about a Columbia University professor who has early onset Alzheimer's disease, based on Lisa Genova's 2007 novel of the same name and featuring Julianne Moore in the title role. Documentaries on Alzheimer's disease include Malcolm and Barbara: A Love Story (1999) and Malcolm and Barbara: Love's Farewell (2007), both featuring Malcolm Pointon.[269][270][271]
Alzheimer's disease has also been portrayed in music by English musician the Caretaker in releases such as Persistent Repetition of Phrases (2008), An Empty Bliss Beyond This World (2011), and Everywhere at the End of Time (2016–2019).[272][273][274] Paintings depicting the disorder include the late works by American artist William Utermohlen, who drew self-portraits from 1995 to 2000 as an experiment of showing his disease through art.[275][276]
Research directions
Antibodies may have the ability to alter the disease course by targeting amyloid beta with immunotherapy medications such as donanemab, aducanumab, and lecanemab.[277][278][279] Aducanumab was approved by the US Food and Drug Administration (FDA) in 2021, using the accelerated approval process, although the approval generated controversy and more evidence is needed to address administration, safety, and effectiveness.[280][281][282][283] It has less effectiveness in people who already had severe Alzheimer's symptoms.[284] In early 2024, Biogen announced it would discontinue aducanumab.[285]
Lecanemab, which clears plaques and reduces amyloid proteins,[286] was approved via the FDA accelerated approval process,[287][288][289] and was converted to traditional approval in July 2023, after further testing, along with the addition of a boxed warning about amyloid-related imaging abnormalities.[290][291] As of early August 2024, lecanemab was approved for sale in Japan, South Korea, China, Hong Kong and Israel although it was recommended against approval by an advisory body of the European Union on July 26, citing its side effects.[285]
Donanemab, which clears plaques,[286] was approved by the FDA in July 2024.[292] Anti-amyloid drugs also cause brain shrinkage.[293] The cholinesterase inhibitor benzgalantamine was approved by the FDA in July 2024.[294]
Specific medications that may reduce the risk or progression of Alzheimer's disease have been studied.[295] The research trials investigating medications generally impact Aβ plaques, inflammation, APOE, neurotransmitter receptors, neurogenesis, growth factors or hormones.[295][296][297]
Machine learning algorithms with electronic health records are being studied as a way to predict Alzheimer's disease earlier.[298]
Focused ultrasound for medication delivery
In 2024, a new technique of using focused ultrasound for the delivery of medication past the blood-brain barrier (BBB) is being tested. At the point where the ultrasound beams converge, the focused ultrasound produces several therapeutic effects without incisions or radiation. This can cause opening of the blood-brain barrier (BBB), which may aid in the removal of beta amyloid or tao from the brain.[299]
References
- ^ a b c d e f g h i j k Knopman DS, Amieva H, Petersen RC, Chételat G, Holtzman DM, Hyman BT, et al. (May 2021). "Alzheimer disease". Nature Reviews Disease Primers. 7 (1): 33. doi:10.1038/s41572-021-00269-y. PMC 8574196. PMID 33986301.
- ^ a b c d e f "Dementia Fact sheet". World Health Organization. 15 March 2023. Retrieved 10 July 2023.
- ^ a b c "Ask the Doctors - What is the cause of death in Alzheimer's disease?". www.uclahealth.org. Retrieved 18 March 2024.
- ^ a b Mendez MF (November 2012). "Early-onset Alzheimer's disease: nonamnestic subtypes and type 2 AD". Archives of Medical Research. 43 (8): 677–685. doi:10.1016/j.arcmed.2012.11.009. PMC 3532551. PMID 23178565.
- ^ a b c d e f g h i Yu JT, Xu W, Tan CC, Andrieu S, Suckling J, Evangelou E, et al. (November 2020). "Evidence-based prevention of Alzheimer's disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials". Journal of Neurology, Neurosurgery, and Psychiatry. 91 (11): 1201–1209. doi:10.1136/jnnp-2019-321913. PMC 7569385. PMID 32690803.
- ^ a b Cheng ST (September 2016). "Cognitive Reserve and the Prevention of Dementia: the Role of Physical and Cognitive Activities". Current Psychiatry Reports (Review). 18 (9): 85. doi:10.1007/s11920-016-0721-2. PMC 4969323. PMID 27481112.
- ^ a b c Viña J, Sanz-Ros J (October 2018). "Alzheimer's disease: Only prevention makes sense". European Journal of Clinical Investigation (Review). 48 (10): e13005. doi:10.1111/eci.13005. PMID 30028503. S2CID 51703879.
- ^ a b "Dementia diagnosis and assessment" (PDF). National Institute for Health and Care Excellence (NICE). Archived from the original (PDF) on 5 December 2014. Retrieved 30 November 2014.
- ^ Gomperts SN (April 2016). "Lewy Body Dementias: Dementia With Lewy Bodies and Parkinson Disease Dementia". Continuum (Review). 22 (2 Dementia): 435–463. doi:10.1212/CON.0000000000000309. ISSN 1080-2371. PMC 5390937. PMID 27042903.
- ^ a b Lott IT, Head E (March 2019). "Dementia in Down syndrome: unique insights for Alzheimer disease research". Nat Rev Neurol. 15 (3): 135–147. doi:10.1038/s41582-018-0132-6. PMC 8061428. PMID 30733618.
- ^ a b c "How Alzheimer's drugs help manage symptoms". Mayo Clinic. 30 August 2023. Retrieved 19 March 2024.
- ^ a b Schaffert J, LoBue C, Hynan LS, Hart J, Rossetti H, Carlew AR, et al. (2022). "Predictors of Life Expectancy in Autopsy-Confirmed Alzheimer's Disease". Journal of Alzheimer's Disease. 86 (1): 271–281. doi:10.3233/JAD-215200. PMC 8966055. PMID 35034898.
- ^ a b Todd S, Barr S, Roberts M, Passmore AP (November 2013). "Survival in dementia and predictors of mortality: a review". International Journal of Geriatric Psychiatry. 28 (11): 1109–1124. doi:10.1002/gps.3946. PMID 23526458.
- ^ a b c d e f g h i j k Breijyeh Z, Karaman R (December 2020). "Comprehensive Review on Alzheimer's Disease: Causes and Treatment". Molecules (Review). 25 (24): 5789. doi:10.3390/molecules25245789. PMC 7764106. PMID 33302541.
- ^ a b c d Simon RP, Greenberg DA, Aminoff MJ (2018). Clinical neurology (Tenth ed.). [New York]: McGraw Hill. p. 111. ISBN 978-1-259-86173-4. OCLC 1012400314.
- ^ a b c d Burns A, Iliffe S (February 2009). "Alzheimer's disease". BMJ. 338: b158. doi:10.1136/bmj.b158. PMID 19196745. S2CID 8570146.
- ^ a b c d Long JM, Holtzman DM (October 2019). "Alzheimer Disease: An Update on Pathobiology and Treatment Strategies". Cell. 179 (2): 312–339. doi:10.1016/j.cell.2019.09.001. PMC 6778042. PMID 31564456.
- ^ a b "Study reveals how APOE4 gene may increase risk for dementia". National Institute on Aging. 16 March 2021. Archived from the original on 17 March 2021. Retrieved 17 March 2021.
- ^ a b c d "Alzheimer's Disease Fact Sheet". National Institute on Aging. Archived from the original on 23 March 2022. Retrieved 23 March 2022.
- ^ Dementia: assessment, management and support for people living with dementia and their carers (Report). National Institute for Health and Care Excellence (NICE). 20 June 2018. NG97. Retrieved 8 July 2023.
- ^ a b Khan S, Barve KH, Kumar MS (2020). "Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer's Disease". Curr Neuropharmacol. 18 (11): 1106–1125. doi:10.2174/1570159X18666200528142429. PMC 7709159. PMID 32484110.
- ^ a b c Gauthreaux K, Bonnett TA, Besser LM, Brenowitz WD, Teylan M, Mock C, et al. (May 2020). "Concordance of Clinical Alzheimer Diagnosis and Neuropathological Features at Autopsy". Journal of Neuropathology and Experimental Neurology. 79 (5): 465–473. doi:10.1093/jnen/nlaa014. PMC 7160616. PMID 32186726.
- ^ a b c d Thompson CA, Spilsbury K, Hall J, Birks Y, Barnes C, Adamson J (July 2007). "Systematic review of information and support interventions for caregivers of people with dementia". BMC Geriatrics. 7: 18. doi:10.1186/1471-2318-7-18. PMC 1951962. PMID 17662119.
- ^ Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S (April 2015). "Exercise programs for people with dementia". The Cochrane Database of Systematic Reviews (Submitted manuscript). 132 (4): CD006489. doi:10.1002/14651858.CD006489.pub4. PMC 9426996. PMID 25874613.
- ^ "Low-dose antipsychotics in people with dementia". National Institute for Health and Care Excellence (NICE). Archived from the original on 5 December 2014. Retrieved 29 November 2014.
- ^ "Information for Healthcare Professionals: Conventional Antipsychotics". US Food and Drug Administration. 16 June 2008. Archived from the original on 29 November 2014. Retrieved 29 November 2014.
- ^ a b "Alzheimer's Disease Fact Sheet". National Institute on Aging. Archived from the original on 24 January 2021. Retrieved 25 January 2021.
- ^ Zhu D, Montagne A, Zhao Z (June 2021). "Alzheimer's pathogenic mechanisms and underlying sex difference". Cell Mol Life Sci. 78 (11): 4907–4920. doi:10.1007/s00018-021-03830-w. PMC 8720296. PMID 33844047.
- ^ a b c Berchtold NC, Cotman CW (1998). "Evolution in the conceptualization of dementia and Alzheimer's disease: Greco-Roman period to the 1960s". Neurobiology of Aging. 19 (3): 173–189. doi:10.1016/S0197-4580(98)00052-9. PMID 9661992. S2CID 24808582.
- ^ "The top 10 causes of death". www.who.int. Retrieved 19 March 2024.
- ^ Bertagnolli MM (5 August 2024). "Fiscal Year 2026 NIH Professional Judgment Budget for Alzheimer's Disease and Related Dementias Research: Advancing Progress in Dementia Research". US National Institutes of Health. Retrieved 23 September 2024.
- ^ "Alzheimer Europe: Horizon Europe research programme".
- ^ a b c "Alzheimer's disease – Symptoms". National Health Service (NHS). 10 May 2018. Archived from the original on 30 January 2021. Retrieved 25 January 2021.
- ^ a b Waldemar G, Dubois B, Emre M, Georges J, McKeith IG, Rossor M, et al. (January 2007). "Recommendations for the diagnosis and management of Alzheimer's disease and other disorders associated with dementia: EFNS guideline". European Journal of Neurology. 14 (1): e1-26. doi:10.1111/j.1468-1331.2006.01605.x. PMID 17222085. S2CID 2725064.
- ^ a b c Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ (September 2004). "Multiple cognitive deficits during the transition to Alzheimer's disease". Journal of Internal Medicine. 256 (3): 195–204. doi:10.1111/j.1365-2796.2004.01386.x. PMID 15324363. S2CID 37005854.
- ^ Nygård L (2003). "Instrumental activities of daily living: a stepping-stone towards Alzheimer's disease diagnosis in subjects with mild cognitive impairment?". Acta Neurologica Scandinavica. Supplementum. 179 (s179): 42–46. doi:10.1034/j.1600-0404.107.s179.8.x. PMID 12603250. S2CID 25313065.
- ^ Deardorff WJ, Grossberg GT (2019). "Behavioral and psychological symptoms in Alzheimer's dementia and vascular dementia". Psychopharmacology of Neurologic Disease. Handbook of Clinical Neurology. Vol. 165. Elsevier. pp. 5–32. doi:10.1016/B978-0-444-64012-3.00002-2. ISBN 978-0-444-64012-3. PMID 31727229. S2CID 208037448.
- ^ Murray ED, Buttner N, Price BH (2012). "Depression and Psychosis in Neurological Practice". In Bradley WG, Daroff RB, Fenichel GM, Jankovic J (eds.). Bradley's neurology in clinical practice (6th ed.). Philadelphia, PA: Elsevier/Saunders. ISBN 978-1-4377-0434-1.
- ^ a b Petersen RC, Lopez O, Armstrong MJ, Getchius TS, Ganguli M, Gloss D, et al. (January 2018). "Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology". Neurology. 90 (3): 126–135. doi:10.1212/WNL.0000000000004826. PMC 5772157. PMID 29282327.
- ^ a b c Atri A (March 2019). "The Alzheimer's Disease Clinical Spectrum: Diagnosis and Management". The Medical Clinics of North America (Review). 103 (2): 263–293. doi:10.1016/j.mcna.2018.10.009. PMID 30704681. S2CID 73432842.
- ^ a b c d e f g h i j k l m n o p q r s Förstl H, Kurz A (1999). "Clinical features of Alzheimer's disease". European Archives of Psychiatry and Clinical Neuroscience. 249 (6): 288–290. doi:10.1007/s004060050101. PMID 10653284. S2CID 26142779.
- ^ Carlesimo GA, Oscar-Berman M (June 1992). "Memory deficits in Alzheimer's patients: a comprehensive review". Neuropsychology Review. 3 (2): 119–169. doi:10.1007/BF01108841. PMID 1300219. S2CID 19548915.
- ^ Jelicic M, Bonebakker AE, Bonke B (1995). "Implicit memory performance of patients with Alzheimer's disease: a brief review". International Psychogeriatrics. 7 (3): 385–392. doi:10.1017/S1041610295002134. PMID 8821346. S2CID 9419442.
- ^ a b Taler V, Phillips NA (July 2008). "Language performance in Alzheimer's disease and mild cognitive impairment: a comparative review". Journal of Clinical and Experimental Neuropsychology. 30 (5): 501–556. doi:10.1080/13803390701550128. PMID 18569251. S2CID 37153159.
- ^ a b c Frank EM (September 1994). "Effect of Alzheimer's disease on communication function". Journal of the South Carolina Medical Association. 90 (9): 417–423. PMID 7967534.
- ^ Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A (May 2001). "Sundowning and circadian rhythms in Alzheimer's disease". The American Journal of Psychiatry. 158 (5): 704–711. doi:10.1176/appi.ajp.158.5.704. PMID 11329390. S2CID 10492607.
- ^ Gold DP, Reis MF, Markiewicz D, Andres D (January 1995). "When home caregiving ends: a longitudinal study of outcomes for caregivers of relatives with dementia". Journal of the American Geriatrics Society. 43 (1): 10–16. doi:10.1111/j.1532-5415.1995.tb06235.x. PMID 7806732. S2CID 29847950.
- ^ Mashour GA, Frank L, Batthyany A, Kolanowski AM, Nahm M, Schulman-Green D, et al. (August 2019). "Paradoxical lucidity: A potential paradigm shift for the neurobiology and treatment of severe dementias". Alzheimer's & Dementia. 15 (8): 1107–1114. doi:10.1016/j.jalz.2019.04.002. hdl:2027.42/153062. PMID 31229433. S2CID 195063786.
- ^ "Alzheimer's disease – Causes". National Health Service (NHS). 24 April 2023. Archived from the original on 29 September 2020. Retrieved 10 July 2023.
- ^ Tackenberg C, Kulic L, Nitsch RM (2020). "Familial Alzheimer's disease mutations at position 22 of the amyloid β-peptide sequence differentially affect synaptic loss, tau phosphorylation and neuronal cell death in an ex vivo system". PLOS ONE. 15 (9): e0239584. Bibcode:2020PLoSO..1539584T. doi:10.1371/journal.pone.0239584. PMC 7510992. PMID 32966331.
- ^ Wang H, Kulas JA, Wang C, Holtzman DM, Ferris HA, Hansen SB (August 2021). "Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol". Proceedings of the National Academy of Sciences of the United States of America. 118 (33): e2102191118. Bibcode:2021PNAS..11802191W. doi:10.1073/pnas.2102191118. ISSN 0027-8424. PMC 8379952. PMID 34385305. S2CID 236998499.
- ^ Vilchez D, Saez I, Dillin A (December 2014). "The role of protein clearance mechanisms in organismal ageing and age-related diseases". Nature Communications. 5: 5659. Bibcode:2014NatCo...5.5659V. doi:10.1038/ncomms6659. PMID 25482515.
- ^ Jacobson M, McCarthy N (2002). Apoptosis. Oxford, OX: Oxford University Press. p. 290. ISBN 0-19-963849-7.
- ^ Hardy J, Allsop D (October 1991). "Amyloid deposition as the central event in the aetiology of Alzheimer's disease". Trends in Pharmacological Sciences. 12 (10): 383–388. doi:10.1016/0165-6147(91)90609-V. PMID 1763432.
- ^ Mudher A, Lovestone S (January 2002). "Alzheimer's disease-do tauists and baptists finally shake hands?". Trends in Neurosciences. 25 (1): 22–26. doi:10.1016/S0166-2236(00)02031-2. PMID 11801334. S2CID 37380445.
- ^ Polvikoski T, Sulkava R, Haltia M, Kainulainen K, Vuorio A, Verkkoniemi A, et al. (November 1995). "Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein". The New England Journal of Medicine. 333 (19): 1242–1247. doi:10.1056/NEJM199511093331902. PMID 7566000.
- ^ a b Andrews SJ, Renton AE, Fulton-Howard B, Podlesny-Drabiniok A, Marcora E, Goate AM (April 2023). "The complex genetic architecture of Alzheimer's disease: novel insights and future directions". eBioMedicine. 90: 104511. doi:10.1016/j.ebiom.2023.104511. PMC 10024184. PMID 36907103.
- ^ a b Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. (April 2021). "Alzheimer's disease". Lancet. 397 (10284): 1577–1590. doi:10.1016/S0140-6736(20)32205-4. PMC 8354300. PMID 33667416.
- ^ Sims R, Hill M, Williams J (March 2020). "The multiplex model of the genetics of Alzheimer's disease" (PDF). Nat Neurosci. 23 (3): 311–322. doi:10.1038/s41593-020-0599-5. PMID 32112059. S2CID 256839971.
- ^ Chávez-Gutiérrez L, Szaruga M (1 September 2020). "Mechanisms of neurodegeneration — Insights from familial Alzheimer's disease". Seminars in Cell & Developmental Biology. Gamma Secretase. 105: 75–85. doi:10.1016/j.semcdb.2020.03.005. ISSN 1084-9521. PMID 32418657.
- ^ Piaceri I, Nacmias B, Sorbi S (January 2013). "Genetics of familial and sporadic Alzheimer's disease". Frontiers in Bioscience (Elite Edition). 5 (1): 167–177. doi:10.2741/e605. PMID 23276979.
- ^ Perea JR, Bolós M, Avila J (October 2020). "Microglia in Alzheimer's Disease in the Context of Tau Pathology". Biomolecules. 10 (10): 1439. doi:10.3390/biom10101439. PMC 7602223. PMID 33066368.
- ^ Mahley RW, Weisgraber KH, Huang Y (April 2006). "Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease". Proceedings of the National Academy of Sciences of the United States of America. 103 (15): 5644–5651. Bibcode:2006PNAS..103.5644M. doi:10.1073/pnas.0600549103. PMC 1414631. PMID 16567625.
- ^ Blennow K, de Leon MJ, Zetterberg H (July 2006). "Alzheimer's disease". Lancet. 368 (9533): 387–403. doi:10.1016/S0140-6736(06)69113-7. PMID 16876668. S2CID 47544338.
- ^ Hall K, Murrell J, Ogunniyi A, Deeg M, Baiyewu O, Gao S, et al. (January 2006). "Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba". Neurology. 66 (2): 223–227. doi:10.1212/01.wnl.0000194507.39504.17. PMC 2860622. PMID 16434658.
- ^ Gureje O, Ogunniyi A, Baiyewu O, Price B, Unverzagt FW, Evans RM, et al. (January 2006). "APOE epsilon4 is not associated with Alzheimer's disease in elderly Nigerians". Annals of Neurology. 59 (1): 182–185. doi:10.1002/ana.20694. PMC 2855121. PMID 16278853.
- ^ Schramm C, Wallon D, Nicolas G, Charbonnier C (1 May 2022). "What contribution can genetics make to predict the risk of Alzheimer's disease?". Revue Neurologique. International meeting of the French society of neurology : NeuroDegenerative Disease : What will the future bring ?. 178 (5): 414–421. doi:10.1016/j.neurol.2022.03.005. ISSN 0035-3787. PMID 35491248.
- ^ Goldman JS, Van Deerlin VM (1 October 2018). "Alzheimer's Disease and Frontotemporal Dementia: The Current State of Genetics and Genetic Testing Since the Advent of Next-Generation Sequencing". Molecular Diagnosis & Therapy. 22 (5): 505–513. doi:10.1007/s40291-018-0347-7. ISSN 1179-2000. PMC 6472481. PMID 29971646.
- ^ Piaceri I (2013). "Genetics of familial and sporadic Alzheimer s disease". Frontiers in Bioscience. E5 (1): 167–177. doi:10.2741/E605. ISSN 1945-0494. PMID 23276979.
- ^ Selkoe DJ (June 1999). "Translating cell biology into therapeutic advances in Alzheimer's disease". Nature. 399 (6738 Suppl): A23–A31. doi:10.1038/19866. PMID 10392577. S2CID 42287088.
- ^ Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, et al. (November 1996). "Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo". Neuron. 17 (5): 1005–1013. doi:10.1016/S0896-6273(00)80230-5. PMID 8938131. S2CID 18315650.
- ^ Kim JH (December 2018). "Genetics of Alzheimer's Disease". Dementia and Neurocognitive Disorders. 17 (4): 131–136. doi:10.12779/dnd.2018.17.4.131. PMC 6425887. PMID 30906402.
- ^ Carmona S, Zahs K, Wu E, Dakin K, Bras J, Guerreiro R (August 2018). "The role of TREM2 in Alzheimer's disease and other neurodegenerative disorders". Lancet Neurol. 17 (8): 721–730. doi:10.1016/S1474-4422(18)30232-1. PMID 30033062. S2CID 51706988. Archived from the original on 27 March 2022. Retrieved 21 February 2022.
- ^ Tomiyama T (July 2010). "[Involvement of beta-amyloid in the etiology of Alzheimer's disease]". Brain and Nerve = Shinkei Kenkyu No Shinpo. 62 (7): 691–699. PMID 20675873.
- ^ Tomiyama T, Nagata T, Shimada H, Teraoka R, Fukushima A, Kanemitsu H, et al. (March 2008). "A new amyloid beta variant favoring oligomerization in Alzheimer's-type dementia". Annals of Neurology. 63 (3): 377–387. doi:10.1002/ana.21321. PMID 18300294. S2CID 42311988.
- ^ Tomiyama T, Shimada H (February 2020). "APP Osaka Mutation in Familial Alzheimer's Disease-Its Discovery, Phenotypes, and Mechanism of Recessive Inheritance". International Journal of Molecular Sciences. 21 (4): 1413. doi:10.3390/ijms21041413. PMC 7073033. PMID 32093100.
- ^ a b c d e f g h Tzioras M, Davies C, Newman A, Jackson R, Spires-Jones T (June 2019). "Invited Review: APOE at the interface of inflammation, neurodegeneration and pathological protein spread in Alzheimer's disease". Neuropathology and Applied Neurobiology. 45 (4): 327–346. doi:10.1111/nan.12529. PMC 6563457. PMID 30394574.
- ^ Sinyor B, Mineo J, Ochner C (June 2020). "Alzheimer's Disease, Inflammation, and the Role of Antioxidants". Journal of Alzheimer's Disease Reports. 4 (1): 175–183. doi:10.3233/ADR-200171. PMC 7369138. PMID 32715278.
- ^ Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT (2018). "Inflammation as a central mechanism in Alzheimer's disease". Alzheimer's & Dementia. 4: 575–590. doi:10.1016/j.trci.2018.06.014. PMC 6214864. PMID 30406177.
- ^ Lin X, Kapoor A, Gu Y, Chow MJ, Peng J, Zhao K, et al. (February 2020). "Contributions of DNA Damage to Alzheimer's Disease". Int J Mol Sci. 21 (5): 1666. doi:10.3390/ijms21051666. PMC 7084447. PMID 32121304.
- ^ Irwin MR, Vitiello MV (March 2019). "Implications of sleep disturbance and inflammation for Alzheimer's disease dementia". The Lancet. Neurology. 18 (3): 296–306. doi:10.1016/S1474-4422(18)30450-2. PMID 30661858. S2CID 58546748.
- ^ Lloret MA, Cervera-Ferri A, Nepomuceno M, Monllor P, Esteve D, Lloret A (February 2020). "Is Sleep Disruption a Cause or Consequence of Alzheimer's Disease? Reviewing Its Possible Role as a Biomarker". International Journal of Molecular Sciences. 21 (3): 1168. doi:10.3390/ijms21031168. PMC 7037733. PMID 32050587.
- ^ Huat TJ, Camats-Perna J, Newcombe EA, Valmas N, Kitazawa M, Medeiros R (April 2019). "Metal Toxicity Links to Alzheimer's Disease and Neuroinflammation". J Mol Biol. 431 (9): 1843–1868. doi:10.1016/j.jmb.2019.01.018. PMC 6475603. PMID 30664867.
- ^ Eikelenboom P, van Exel E, Hoozemans JJ, Veerhuis R, Rozemuller AJ, van Gool WA (2010). "Neuroinflammation – an early event in both the history and pathogenesis of Alzheimer's disease". Neuro-Degenerative Diseases. 7 (1–3): 38–41. doi:10.1159/000283480. PMID 20160456. S2CID 40048333.
- ^ Alves GS, Oertel Knöchel V, Knöchel C, Carvalho AF, Pantel J, Engelhardt E, et al. (2015). "Integrating retrogenesis theory to Alzheimer's disease pathology: insight from DTI-TBSS investigation of the white matter microstructural integrity". BioMed Research International. 2015: 291658. doi:10.1155/2015/291658. PMC 4320890. PMID 25685779.
- ^ Reisberg B, Franssen EH, Hasan SM, Monteiro I, Boksay I, Souren LE, et al. (1999). "Retrogenesis: clinical, physiologic, and pathologic mechanisms in brain aging, Alzheimer's and other dementing processes". European Archives of Psychiatry and Clinical Neuroscience. 249 (3): 28–36. doi:10.1007/pl00014170. PMID 10654097. S2CID 23410069.
- ^ Bartzokis G (August 2011). "Alzheimer's disease as homeostatic responses to age-related myelin breakdown". Neurobiology of Aging. 32 (8): 1341–1371. doi:10.1016/j.neurobiolaging.2009.08.007. PMC 3128664. PMID 19775776.
- ^ Cai Z, Xiao M (2016). "Oligodendrocytes and Alzheimer's disease". The International Journal of Neuroscience. 126 (2): 97–104. doi:10.3109/00207454.2015.1025778. PMID 26000818. S2CID 21448714.
- ^ Depp C, Sun T, Sasmita AO, Spieth L, Berghoff SA, Nazarenko T, et al. (June 2023). "Myelin dysfunction drives amyloid-β deposition in models of Alzheimer's disease". Nature. 618 (7964): 349–357. Bibcode:2023Natur.618..349D. doi:10.1038/s41586-023-06120-6. PMC 10247380. PMID 37258678.
- ^ Luczynski P, Laule C, Hsiung GR, Moore GR, Tremlett H (January 2019). "Coexistence of Multiple Sclerosis and Alzheimer's disease: A review". Multiple Sclerosis and Related Disorders. 27: 232–238. doi:10.1016/j.msard.2018.10.109. PMID 30415025.
- ^ Londoño DP, Arumaithurai K, Constantopoulos E, Basso MR, Reichard RR, Flanagan EP, et al. (4 July 2022). "Diagnosis of coexistent neurodegenerative dementias in multiple sclerosis". Brain Communications. 4 (4): fcac167. doi:10.1093/braincomms/fcac167. PMC 9272064. PMID 35822102.
- ^ Zis P, Hadjivassiliou M (February 2019). "Treatment of Neurological Manifestations of Gluten Sensitivity and Coeliac Disease". Current Treatment Options in Neurology. 21 (3): 10. doi:10.1007/s11940-019-0552-7. PMID 30806821. S2CID 73466457.
- ^ Makhlouf S, Messelmani M, Zaouali J, Mrissa R (March 2018). "Cognitive impairment in celiac disease and non-celiac gluten sensitivity: review of literature on the main cognitive impairments, the imaging and the effect of gluten free diet". Acta Neurologica Belgica (Review). 118 (1): 21–27. doi:10.1007/s13760-017-0870-z. PMID 29247390. S2CID 3943047.
- ^ Zhou L, Miranda-Saksena M, Saksena NK (May 2013). "Viruses and neurodegeneration". Virology Journal. 10 (1): 172. doi:10.1186/1743-422X-10-172. PMC 3679988. PMID 23724961.
- ^ Gonzalez-Fernandez E, Huang J (September 2023). "Cognitive Aspects of COVID-19". Current Neurology and Neuroscience Reports. 23 (9): 531–538. doi:10.1007/s11910-023-01286-y. PMID 37490194. S2CID 260132167.
- ^ Wenk GL (2003). "Neuropathologic changes in Alzheimer's disease". The Journal of Clinical Psychiatry. 64 (Suppl 9): 7–10. PMID 12934968.
- ^ Braak H, Del Tredici K (December 2012). "Where, when, and in what form does sporadic Alzheimer's disease begin?". Current Opinion in Neurology. 25 (6): 708–714. doi:10.1097/WCO.0b013e32835a3432. PMID 23160422.
- ^ Desikan RS, Cabral HJ, Hess CP, Dillon WP, Glastonbury CM, Weiner MW, et al. (August 2009). "Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease". Brain. 132 (Pt 8): 2048–2057. doi:10.1093/brain/awp123. PMC 2714061. PMID 19460794.
- ^ Moan R (July 2009). "MRI Software Accurately IDs Preclinical Alzheimer's Disease". Diagnostic Imaging. Archived from the original on 21 February 2022. Retrieved 21 February 2022.
- ^ Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J (June 2004). "The importance of neuritic plaques and tangles to the development and evolution of AD". Neurology. 62 (11): 1984–1989. doi:10.1212/01.WNL.0000129697.01779.0A. PMID 15184601. S2CID 25017332.
- ^ DeTure MA, Dickson DW (August 2019). "The neuropathological diagnosis of Alzheimer's disease". Molecular Neurodegeneration. 14 (1): 32. doi:10.1186/s13024-019-0333-5. PMC 6679484. PMID 31375134.
- ^ Tiraboschi P, Sabbagh MN, Hansen LA, Salmon DP, Merdes A, Gamst A, et al. (April 2004). "Alzheimer disease without neocortical neurofibrillary tangles: "a second look"". Neurology. 62 (7): 1141–1147. doi:10.1212/01.wnl.0000118212.41542.e7. PMID 15079014. S2CID 22832110.
- ^ Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH (1994). "Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital". Cerebral Cortex. 4 (2): 138–150. doi:10.1093/cercor/4.2.138. PMID 8038565.
- ^ Kotzbauer PT, Trojanowsk JQ, Lee VM (October 2001). "Lewy body pathology in Alzheimer's disease". Journal of Molecular Neuroscience. 17 (2): 225–232. doi:10.1385/JMN:17:2:225. PMID 11816795. S2CID 44407971.
- ^ Hernández F, Avila J (September 2007). "Tauopathies". Cellular and Molecular Life Sciences. 64 (17): 2219–2233. doi:10.1007/s00018-007-7220-x. PMC 11136052. PMID 17604998. S2CID 261121643.
- ^ Sun W, Samimi H, Gamez M, Zare H, Frost B (August 2018). "Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies". Nature Neuroscience. 21 (8): 1038–1048. doi:10.1038/s41593-018-0194-1. PMC 6095477. PMID 30038280.
- ^ Balusu S, Horré K, Thrupp N, Craessaerts K, Snellinx A, Serneels L, T'Syen D, Chrysidou I, Arranz AM, Sierksma A, Simrén J, Karikari TK, Zetterberg H, Chen WT, Thal DR, Salta E, Fiers M, De Strooper B. MEG3 activates necroptosis in human neuron xenografts modeling Alzheimer's disease. Science. 2023 Sep 15;381(6663):1176-1182. doi:10.1126/science.abp9556 PMID 37708272
- ^ "Scientists discover how brain cells die in Alzheimer's". BBC News. 15 September 2023. Retrieved 27 September 2023.
- ^ Van Broeck B, Van Broeckhoven C, Kumar-Singh S (2007). "Current insights into molecular mechanisms of Alzheimer disease and their implications for therapeutic approaches". Neuro-Degenerative Diseases. 4 (5): 349–365. doi:10.1159/000105156. PMID 17622778. S2CID 7949658.
- ^ Huang Y, Mucke L (March 2012). "Alzheimer mechanisms and therapeutic strategies". Cell. 148 (6): 1204–1222. doi:10.1016/j.cell.2012.02.040. PMC 3319071. PMID 22424230.
- ^ Yankner BA, Duffy LK, Kirschner DA (October 1990). "Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides". Science. 250 (4978): 279–282. Bibcode:1990Sci...250..279Y. doi:10.1126/science.2218531. PMID 2218531.
- ^ Chen X, Yan SD (December 2006). "Mitochondrial Abeta: a potential cause of metabolic dysfunction in Alzheimer's disease". IUBMB Life. 58 (12): 686–694. doi:10.1080/15216540601047767. PMID 17424907. S2CID 85423830.
- ^ Ryan SK, Ugalde CL, Rolland AS, Skidmore J, Devos D, Hammond TR (October 2023). "Therapeutic inhibition of ferroptosis in neurodegenerative disease". Trends in Pharmacological Sciences. 44 (10): 674–688. doi:10.1016/j.tips.2023.07.007. PMID 37657967.
- ^ Greig NH, Mattson MP, Perry T, Chan SL, Giordano T, Sambamurti K, et al. (December 2004). "New therapeutic strategies and drug candidates for neurodegenerative diseases: p53 and TNF-alpha inhibitors, and GLP-1 receptor agonists". Annals of the New York Academy of Sciences. 1035 (1): 290–315. Bibcode:2004NYASA1035..290G. doi:10.1196/annals.1332.018. PMID 15681814. S2CID 84659695. Archived from the original on 3 June 2020. Retrieved 19 July 2019.
- ^ Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. (April 2015). "Neuroinflammation in Alzheimer's disease". The Lancet. Neurology. 14 (4): 388–405. doi:10.1016/S1474-4422(15)70016-5. PMC 5909703. PMID 25792098.
- ^ Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S (November 2008). "New insights into brain BDNF function in normal aging and Alzheimer disease". Brain Research Reviews. 59 (1): 201–220. doi:10.1016/j.brainresrev.2008.07.007. hdl:10533/142174. PMID 18708092. S2CID 6589846.
- ^ Schindowski K, Belarbi K, Buée L (February 2008). "Neurotrophic factors in Alzheimer's disease: role of axonal transport". Genes, Brain and Behavior. 7 (Suppl 1): 43–56. doi:10.1111/j.1601-183X.2007.00378.x. PMC 2228393. PMID 18184369.
- ^ a b Sachdev PS, Blacker D, Blazer DG, Ganguli M, Jeste DV, Paulsen JS, et al. (November 2014). "Classifying neurocognitive disorders: the DSM-5 approach". Nature Reviews. Neurology. 10 (11): 634–642. doi:10.1038/nrneurol.2014.181. PMID 25266297. S2CID 20635070. Archived from the original on 20 March 2022. Retrieved 27 November 2021.
- ^ a b c d Arvanitakis Z, Shah RC, Bennett DA (22 October 2019). "Diagnosis and Management of Dementia: Review". JAMA. 322 (16): 1589–1599. doi:10.1001/jama.2019.4782. PMC 7462122. PMID 31638686.
- ^ Mendez MF (2006). "The accurate diagnosis of early-onset dementia". International Journal of Psychiatry in Medicine. 36 (4): 401–412. doi:10.2190/Q6J4-R143-P630-KW41. PMID 17407994. S2CID 43715976. Archived from the original on 3 June 2020. Retrieved 25 May 2020.
- ^ Klafki HW, Staufenbiel M, Kornhuber J, Wiltfang J (November 2006). "Therapeutic approaches to Alzheimer's disease". Brain. 129 (Pt 11): 2840–2855. doi:10.1093/brain/awl280. PMID 17018549.
- ^ Dementia: Quick Reference Guide (PDF). London: (UK) National Institute for Health and Clinical Excellence. 2006. ISBN 978-1-84629-312-2. Archived from the original (PDF) on 27 February 2008. Retrieved 22 February 2008.
- ^ Schroeter ML, Stein T, Maslowski N, Neumann J (October 2009). "Neural correlates of Alzheimer's disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients". NeuroImage. 47 (4): 1196–1206. doi:10.1016/j.neuroimage.2009.05.037. PMC 2730171. PMID 19463961.
- ^ Jie CV, Treyer V, Schibli R, Mu L (January 2021). "Tauvid: The First FDA-Approved PET Tracer for Imaging Tau Pathology in Alzheimer's Disease". Pharmaceuticals. 14 (2): 110. doi:10.3390/ph14020110. PMC 7911942. PMID 33573211.
- ^ a b c d e Weller J, Budson A (2018). "Current understanding of Alzheimer's disease diagnosis and treatment". F1000Research (Review). 7: 1161. doi:10.12688/f1000research.14506.1. PMC 6073093. PMID 30135715.
- ^ Silva MV, Loures CM, Alves LC, de Souza LC, Borges KB, Carvalho MD (May 2019). "Alzheimer's disease: risk factors and potentially protective measures". Journal of Biomedical Science. 26 (1): 33. doi:10.1186/s12929-019-0524-y. PMC 6507104. PMID 31072403.
- ^ Hane FT, Robinson M, Lee BY, Bai O, Leonenko Z, Albert MS (2017). "Recent Progress in Alzheimer's Disease Research, Part 3: Diagnosis and Treatment". Journal of Alzheimer's Disease (Review). 57 (3): 645–665. doi:10.3233/JAD-160907. PMC 5389048. PMID 28269772.
- ^ Diagnostic and statistical manual of mental disorders: DSM-IV-TR (4th Text Revision ed.). Washington, DC: American Psychiatric Association. 2000. ISBN 978-0-89042-025-6.
- ^ a b c Diagnostic and statistical manual of mental disorders: DSM-5. Washington, D.C.: American Psychiatric Association. 2013. p. 611. ISBN 978-0-89042-555-8.
- ^ Sachs-Ericsson N, Blazer DG (January 2015). "The new DSM-5 diagnosis of mild neurocognitive disorder and its relation to research in mild cognitive impairment". Aging & Mental Health. 19 (1): 2–12. doi:10.1080/13607863.2014.920303. PMID 24914889. S2CID 46244321.
- ^ Stokin GB, Krell-Roesch J, Petersen RC, Geda YE (2015). "Mild Neurocognitive Disorder: An Old Wine in a New Bottle". Harvard Review of Psychiatry (Review). 23 (5): 368–376. doi:10.1097/HRP.0000000000000084. PMC 4894762. PMID 26332219.
- ^ Sperry L, Carlson J, Sauerheber J, Sperry J, eds. (2014). Psychopathology and Psychotherapy: DSM-5 Diagnosis, Case Conceptualization, and Treatment (3 ed.). New York: Routledge. pp. 342–343. doi:10.4324/9780203772287. ISBN 978-0-203-77228-7. Archived from the original on 16 November 2021. Retrieved 16 November 2021.
- ^ Fink HA, Hemmy LS, Linskens EJ, Silverman PC, MacDonald R, McCarten JR, et al. (2020). Diagnosis and Treatment of Clinical Alzheimer's-Type Dementia: A Systematic Review. AHRQ Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US). PMID 32369312. Archived from the original on 7 July 2023. Retrieved 16 November 2021.
- ^ Stokin GB, Krell-Roesch J, Petersen RC, Geda YE (September 2015). "Mild Neurocognitive Disorder: An Old Wine in a New Bottle". Harvard Review of Psychiatry. 23 (5). Wolters Kluwer Health: 368–376. doi:10.1097/HRP.0000000000000084. PMC 4894762. PMID 26332219.
- ^ Bradfield NI, Ames D (April 2020). "Mild cognitive impairment: narrative review of taxonomies and systematic review of their prediction of incident Alzheimer's disease dementia". BJPsych Bulletin (Review). 44 (2): 67–74. doi:10.1192/bjb.2019.77. PMC 7283119. PMID 31724527.
- ^ a b Vega JN, Newhouse PA (October 2014). "Mild cognitive impairment: diagnosis, longitudinal course, and emerging treatments". Current Psychiatry Reports. 16 (10). SpringerLink: 490. doi:10.1007/s11920-014-0490-8. PMC 4169219. PMID 25160795.
- ^ Parnetti L, Chipi E, Salvadori N, D'Andrea K, Eusebi P (January 2019). "Prevalence and risk of progression of preclinical Alzheimer's disease stages: a systematic review and meta-analysis". Alzheimer's Research & Therapy. 11 (1). Springer Nature: 7. doi:10.1186/s13195-018-0459-7. PMC 6334406. PMID 30646955.
- ^ a b c d e Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. (April 2018). "NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease". Alzheimer's & Dementia. 14 (4): 535–562. doi:10.1016/j.jalz.2018.02.018. PMC 5958625. PMID 29653606.
- ^ Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. (May 2011). "Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease". Alzheimer's & Dementia. 7 (3): 280–292. doi:10.1016/j.jalz.2011.03.003. PMC 3220946. PMID 21514248.
- ^ Cheng YW, Chen TF, Chiu MJ (16 February 2017). "From mild cognitive impairment to subjective cognitive decline: conceptual and methodological evolution". Neuropsychiatric Disease and Treatment. 13. Dove Medical Press Limited: 491–498. doi:10.2147/NDT.S123428. PMC 5317337. PMID 28243102.
- ^ Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. (May 2011). "The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease". Alzheimer's & Dementia. 7 (3): 270–279. doi:10.1016/j.jalz.2011.03.008. PMC 3312027. PMID 21514249.
- ^ a b c d Chertkow H, Feldman HH, Jacova C, Massoud F (July 2013). "Definitions of dementia and predementia states in Alzheimer's disease and vascular cognitive impairment: consensus from the Canadian conference on diagnosis of dementia". Alzheimer's Research & Therapy. 5 (Suppl 1). BMC: S2. doi:10.1186/alzrt198. PMC 3981054. PMID 24565215.
- ^ a b c Papadakis MA, McPhee SJ, Rabow MW (2021). Current medical diagnosis & treatment (Sixtieth ed.). New York: McGraw Hill. p. 1760. ISBN 978-1-260-46986-8. OCLC 1195972209.
- ^ Tombaugh TN, McIntyre NJ (September 1992). "The mini-mental state examination: a comprehensive review". Journal of the American Geriatrics Society. 40 (9): 922–935. doi:10.1111/j.1532-5415.1992.tb01992.x. PMID 1512391. S2CID 25169596.
- ^ Pasquier F (January 1999). "Early diagnosis of dementia: neuropsychology". Journal of Neurology. 246 (1): 6–15. doi:10.1007/s004150050299. PMID 9987708. S2CID 2108587.
- ^ Harvey PD, Moriarty PJ, Kleinman L, Coyne K, Sadowsky CH, Chen M, et al. (2005). "The validation of a caregiver assessment of dementia: the Dementia Severity Scale". Alzheimer Disease and Associated Disorders. 19 (4): 186–194. doi:10.1097/01.wad.0000189034.43203.60. PMID 16327345. S2CID 20238911.


 French
French Deutsch
Deutsch