Human papillomavirus infection
| Human papillomavirus infection | |
|---|---|
| Other names | Human papillomavirus |
 | |
| The major capsid protein L1 of HPV 11 | |
| Specialty | Infectious disease, gynecology, oncology |
| Symptoms | None, warts[1][2] |
| Complications | Cancer of the cervix, vulva, vagina, penis, anus, mouth, tonsils, or throat[1][2] [3] |
| Causes | Human papillomavirus spread by direct contact[4][5] |
| Risk factors | Sexual contact |
| Prevention | HPV vaccines, condoms[4][6] |
| Frequency | Most people are infected at some point in time[4] |
Human papillomavirus infection (HPV infection) is caused by a DNA virus from the Papillomaviridae family.[5] Many HPV infections cause no symptoms and 90% resolve spontaneously within two years.[1] In some cases, an HPV infection persists and results in either warts or precancerous lesions.[2] These lesions, depending on the site affected, increase the risk of cancer of the cervix, vulva, vagina, penis, anus, mouth, tonsils, or throat.[1][2][3] Nearly all cervical cancer is due to HPV, and two strains – HPV16 and HPV18 – account for 70% of all cases.[1][7] HPV16 is responsible for almost 90% of HPV-positive oropharyngeal cancers.[3] Between 60% and 90% of the other cancers listed above are also linked to HPV.[7] HPV6 and HPV11 are common causes of genital warts and laryngeal papillomatosis.[1]
An HPV infection is caused by the human papillomavirus, a DNA virus from the papillomavirus family.[8][9] Over 200 types have been described.[10][11] An individual can become infected with more than one type of HPV,[12] and the disease is only known to affect humans.[5][13] More than 40 types may be spread through sexual contact and infect the anus and genitals.[4] Risk factors for persistent infection by sexually transmitted types include early age of first sexual intercourse, multiple sexual partners, smoking, and poor immune function.[1] These types are typically spread by sustained direct skin-to-skin contact, with vaginal and anal sex being the most common methods.[4] HPV infection can also spread from a mother to baby during pregnancy.[12] There is no evidence that HPV can spread via common items like toilet seats,[14] but the types that cause warts may spread via surfaces such as floors.[15] HPV is not killed by common hand sanitizers and disinfectants, increasing the possibility of the virus being transferred via non-living infectious agents called fomites.[16]
HPV vaccines can prevent the most common types of infection.[4] To be most effective, inoculation should occur before the onset of sexual activity, and are therefore recommended between the ages of 9–13 years.[1] For children between the ages of 9–14 years, vaccination effectiveness is reported to range between 74% and 93%, decreasing to 12% to 90% for 15–18 year old adolescents.[17] Cervical cancer screening, such as the Papanicolaou test ("pap smear"), or examination of the cervix after applying acetic acid, can detect both early cancer and abnormal cells that may develop into cancer.[1] Screening allows for early treatment which results in better outcomes.[1] Screening has reduced both the number of cases and the number of deaths from cervical cancer.[18] Genital warts can be removed by freezing.[5]
Nearly every sexually active individual is infected by HPV at some point in their lives.[4] HPV is the most common sexually transmitted infection (STI), globally.[5] High-risk HPVs cause about 5% of all cancers worldwide and about 37,300 cases of cancer in the United States each year.[11] Cervical cancer is among the most common cancers worldwide, causing an estimated 604,000 new cases and 342,000 deaths in 2020.[1] About 90% of these new cases and deaths of cervical cancer occurred in low- and middle-income countries.[1] Roughly 1% of sexually active adults have genital warts.[12] Cases of skin warts have been described since the time of ancient Greece, but it was not until 1907 that they were determined to be caused by a virus.[19]
HPV types
[edit]HPV is a group of more than 200 related viruses, which are designated by a number for each virus type.[11] Some HPV types, such as HPV5, may establish infections that persist for the lifetime of the individual without ever manifesting any clinical symptoms. HPV types 1 and 2 can cause common warts in some infected individuals.[20] HPV types 6 and 11 can cause genital warts and laryngeal papillomatosis.[1]
Many HPV types are carcinogenic.[21] About twelve HPV types (including types 16, 18, 31, and 45) are called "high-risk" types because persistent infection has been linked to cancer of the oropharynx,[3] larynx,[3] vulva, vagina, cervix, penis, and anus.[11][22][23] These cancers all involve sexually transmitted infection of HPV to the stratified epithelial tissue.[1][2] HPV type 16 is the strain most likely to cause cancer and is present in about 47% of all cervical cancers,[24][25] and in many vaginal and vulvar cancers,[26] penile cancers, anal cancers, and cancers of the head and neck.
The table below lists common symptoms of HPV infection and the associated types of HPV.
| Disease | HPV type |
|---|---|
| Common warts | 2, 7, 22 |
| Plantar warts | 1, 2, 4, 63 |
| Flat warts | 3, 10, 28 |
| Anogenital warts | 6, 11, 42, 44 and others[27] |
| Anal dysplasia (lesions) | 16, 18, 31, 53, 58[28] |
| Genital cancers | |
| Epidermodysplasia verruciformis | more than 15 types |
| Focal epithelial hyperplasia (mouth) | 13, 32 |
| Mouth papillomas | 6, 7, 11, 16, 32 |
| Oropharyngeal cancer | 16[3] |
| Verrucous cyst | 60 |
| Laryngeal papillomatosis | 6, 11 |
Available HPV vaccines protect against either two, four, or nine types of HPV.[30] There are six prophylactic HPV vaccines licensed for use: the bivalent vaccines Cervarix, Cecolin, and Walrinvax; the quadrivalent vaccines Cervavax and Gardasil; and the nonavalent vaccine Gardasil 9.[30] All HPV vaccines protect against at least HPV types 16 and 18, which cause the greatest risk of cervical cancer. The quadrivalent vaccines also protect against HPV types 6 and 11. The nonavalent vaccine Gardasil 9 provides protection against those four types (6, 11, 16, and 18), along with five other high-risk HPV types responsible for 20% of cervical cancers (types 31, 33, 45, 52, and 58).[30]
Signs and symptoms
[edit]
Warts
[edit]

Skin infection ("cutaneous" infection) with HPV is very widespread.[31] Skin infections with HPV can cause noncancerous skin growths called warts (verrucae). Warts are caused by the rapid growth of cells on the outer layer of the skin.[32] While cases of warts have been described since the time of ancient Greece, their viral cause was not known until 1907.[19]
Skin warts are most common in childhood and typically appear and regress spontaneously over weeks to months. Recurring skin warts are common.[33] All HPVs are believed to be capable of establishing long-term "latent" infections in small numbers of stem cells present in the skin. Although these latent infections may never be fully eradicated, immunological control is thought to block the appearance of symptoms such as warts. Immunological control is HPV type-specific, meaning an individual may become resistant to one HPV type while remaining susceptible to other types.[citation needed]
Types of warts include:
- Common warts are usually found on the hands and feet, but can also occur in other areas, such as the elbows or knees. Common warts have a characteristic cauliflower-like surface and are typically slightly raised above the surrounding skin. Cutaneous HPV types can cause genital warts but are not associated with the development of cancer.[citation needed]
- Plantar warts are found on the soles of the feet; they grow inward, generally causing pain when walking.
- Subungual or periungual warts form under the fingernail (subungual), around the fingernail, or on the cuticle (periungual). They are more difficult to treat than warts in other locations.[34]
- Flat warts are most commonly found on the arms, face, or forehead. Like common warts, flat warts occur most frequently in children and teens. In people with normal immune function, flat warts are not associated with the development of cancer.[35]
Common, flat, and plantar warts are much less likely to spread from person to person.
Genital warts
[edit]HPV infection of the skin in the genital area is the most common sexually transmitted infection worldwide.[36] Such infections are associated with genital or anal warts (medically known as condylomata acuminata or venereal warts), and these warts are the most easily recognized sign of genital HPV infection.[citation needed]
The strains of HPV that can cause genital warts are usually different from those that cause warts on other parts of the body, such as the hands or feet, or even the inner thighs. A wide variety of HPV types can cause genital warts, but types 6 and 11 together account for about 90% of all cases.[37][38] However, in total more than 40 types of HPV are transmitted through sexual contact and can infect the skin of the anus and genitals.[4] Such infections may cause genital warts, although they may also remain asymptomatic.[citation needed]
The great majority of genital HPV infections never cause any overt symptoms and are cleared by the immune system in a matter of months. Moreover, people may transmit the virus to others even if they do not display overt symptoms of infection. Most people acquire genital HPV infections at some point in their lives, and about 10% of women are currently infected.[36] A large increase in the incidence of genital HPV infection occurs at the age when individuals begin to engage in sexual activity. As with cutaneous HPVs, immunity to genital HPV is believed to be specific to a specific strain of HPV.[citation needed]
Laryngeal papillomatosis
[edit]In addition to genital warts, infection by HPV types 6 and 11 can cause a rare condition known as recurrent laryngeal papillomatosis, in which warts form on the larynx[39] or other areas of the respiratory tract.[40][41] These warts can recur frequently, may interfere with breathing, and in extremely rare cases can progress to cancer. For these reasons, repeated surgery to remove the warts may be advisable.[40][42]
Cancer
[edit]Case statistics
[edit]Cervical cancer is among the most common cancers worldwide, causing an estimated 604,000 new cases and 342,000 deaths in 2020.[1] About 90% of these new cases and deaths of cervical cancer occurred in low- and middle-income countries, where screening tests and treatment of early cervical cell changes are not readily available.[1]
In the United States, about 37,300 cases of cancer due to HPV occur each year.[11]
| Cancer area | Average annual number of cases | HPV attributable (estimated) | HPV 16/18 attributable (estimated) |
|---|---|---|---|
| Cervix | 11,771 | 10,700 | 7,800 |
| Oropharynx (men) | 12,638 | 9,100 | 8,000 |
| Oropharynx (women) | 3,100 | 2,000 | 1,600 |
| Vulva | 3,554 | 2,400 | 1,700 |
| Anus (women) | 3,260 | 3,000 | 2,600 |
| Anus (men) | 1,750 | 1,600 | 1,400 |
| Penis | 1,168 | 700 | 600 |
| Vagina | 802 | 600 | 400 |
| Rectum (women) | 513 | 500 | 400 |
| Rectum (men) | 237 | 200 | 200 |
| Total | 38,793 | 30,700 | 24,600 |
Cancer development
[edit]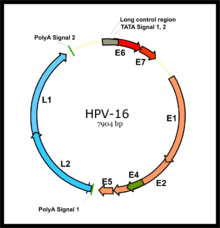
In some infected individuals, their immune systems may fail to control HPV. Lingering infection with high-risk HPV types, such as types 16, 18, 31, and 45, can favor the development of cancer.[44] Co-factors such as cigarette smoke can also enhance the risk of HPV-related cancers.[45][46]
HPV is believed to cause cancer by integrating its genome into nuclear DNA. Some of the early genes expressed by HPV, such as E6 and E7, act as oncogenes that promote tumor growth and malignant transformation.[19] HPV genome integration can also cause carcinogenesis by promoting genomic instability associated with alterations in DNA copy number.[47]
E6 produces a protein (also called E6) that simultaneously binds to two host cell proteins called p53 and E6-Associated Protein (E6-AP). E6AP is an E3 Ubiquitin ligase, an enzyme whose purpose is to tag proteins with a post-translational modification called Ubiquitin. By binding both proteins, E6 induces E6AP to attach a chain of ubiquitin molecules to p53, thereby flagging p53 for proteosomal degradation.[48][49] Normally, p53 acts to prevent cell growth and promotes cell death in the presence of DNA damage. p53 also upregulates the p21 protein, which blocks the formation of the cyclin D/Cdk4 complex, thereby preventing the phosphorylation of retinoblastoma protein (RB), and in turn, halting cell cycle progression by preventing the activation of E2F. In short, p53 is a tumor-suppressor protein that arrests the cell cycle and prevents cell growth and survival when DNA damage occurs.[50] Thus, the degradation of p53, induced by E6, promotes unregulated cell division, cell growth and cell survival, all characteristics of cancer.[51]
It is important to note, that while the interaction between E6, E6AP, and p53 was the first to be characterized, there are multiple other proteins in the host cell which interact with E6 and assist in the induction of cancer.[52]
Squamous cell carcinoma of the skin
[edit]Studies have also shown a link between a wide range of HPV types and squamous cell carcinoma of the skin. In such cases, in vitro studies suggest that the E6 protein of the HPV virus may inhibit apoptosis induced by ultraviolet light.[53]
Cervical cancer
[edit]
Nearly all cases of cervical cancer are associated with HPV infection, with two types, HPV16 and HPV18, present in 70% of cases.[1][7][24][54][55][56] In 2012, twelve HPV types were considered carcinogenic for cervical cancer by the International Agency for Research on Cancer: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59.[57] One study found that 74% of squamous cell carcinomas and 78% of adenocarcinomas tested positive for HPV types 16 or 18.[58] Persistent HPV infection increases the risk for developing cervical carcinoma. Individuals who have an increased incidence of these types of infection are women with HIV/AIDS, who are at a 22-fold increased risk of cervical cancer.[59][60]
The carcinogenic HPV types in cervical cancer belong to the alphapapillomavirus genus and can be grouped further into HPV clades.[61] The two major carcinogenic HPV clades, alphapapillomavirus-9 (A9) and alphapapillomavirus-7 (A7), contain HPV16 and HPV18, respectively.[62] These two HPV clades were shown to have different effects on tumour molecular characteristics and patient prognosis, with clade A7 being associated with more aggressive pathways and an inferior prognosis.[63]
In 2020, about 604,000 new cases and 342,000 deaths from cervical cancer occurred worldwide. Around 90% of these occurred in the developing world.[1]
Most HPV infections of the cervix are cleared rapidly by the immune system and do not progress to cervical cancer (see below the Clearance subsection in Virology). Because the process of transforming normal cervical cells into cancerous ones is slow, cancer occurs in people having been infected with HPV for a long time, usually over a decade or more (persistent infection).[40][64] Furthermore, both the HPV infection and cervical cancer drive metabolic modifications that may be correlated with the aberrant regulation of enzymes related to metabolic pathways.[65]
Non-European (NE) HPV16 variants are significantly more carcinogenic than European (E) HPV16 variants.[66]
Anal cancer
[edit]The risk for anal cancer is 17 to 31 times higher among HIV-positive individuals who were coinfected with high-risk HPV, and 80 times higher for particularly HIV-positive men who have sex with men.[67]
Anal Pap smear screening for anal cancer might benefit some subpopulations of men or women engaging in anal sex.[68] No consensus exists, though, that such screening is beneficial, or who should get an anal Pap smear.[69][70]
Penile cancer
[edit]HPV is associated with approximately 50% of penile cancers. In the United States, penile cancer accounts for about 0.5% of all cancer cases in men. HPV16 is the most commonly associated type detected. The risk of penile cancer increases 2- to 3-fold for individuals who are infected with HIV as well as HPV.[67]
Head and neck cancers
[edit]Oral infection with high-risk carcinogenic HPV types (most commonly HPV 16)[43] is associated with an increasing number of head and neck cancers.[71][55][72][73] This association is independent of tobacco and alcohol use.[73][74][75]
The local percentage varies widely, from 70% in the United States[76] to 4% in Brazil.[77] Engaging in anal or oral sex with an HPV-infected partner may increase the risk of developing these types of cancers.[72]
In the United States, the number of newly diagnosed, HPV-associated head and neck cancers has surpassed that of cervical cancer cases.[71] The rate of such cancers has increased from an estimated 0.8 cases per 100,000 people in 1988[78] to 4.5 per 100,000 in 2012,[43] and, as of 2021, the rate has continued to increase.[79] Researchers explain these recent data by an increase in oral sex. This type of cancer is more common in men than in women.[80]
The mutational profile of HPV-positive and HPV-negative head and neck cancer has been reported, further demonstrating that they are fundamentally distinct diseases.[81]
Lung cancer
[edit]Some evidence links HPV to benign and malignant tumors of the upper respiratory tract. The International Agency for Research on Cancer has found that people with lung cancer were significantly more likely to have several high-risk forms of HPV antibodies compared to those who did not have lung cancer.[82] Researchers looking for HPV among 1,633 lung cancer patients and 2,729 people without the lung disease found that people with lung cancer had more types of HPV than noncancer patients did, and among lung cancer patients, the chances of having eight types of serious HPV were significantly increased.[83] In addition, expression of HPV structural proteins by immunohistochemistry and in vitro studies suggest HPV presence in bronchial cancer and its precursor lesions.[84] Another study detected HPV in the exhaled breath condensate (EBC), bronchial brushing and neoplastic lung tissue of cases, and found a presence of an HPV infection in 16.4% of the subjects affected by nonsmall cell lung cancer, but in none of the controls.[85] The reported average frequencies of HPV in lung cancers were 17% and 15% in Europe and the Americas, respectively, and the mean number of HPV in Asian lung cancer samples was 35.7%, with considerable heterogeneity between certain countries and regions.[86]
Skin cancer
[edit]In very rare cases, HPV may cause epidermodysplasia verruciformis (EV) in individuals with a weakened immune system. The virus, unchecked by the immune system, causes the overproduction of keratin by skin cells, resulting in lesions resembling warts or cutaneous horns which can ultimately transform into skin cancer, but the development is not well understood.[87][88] The specific types of HPV that are associated with EV are HPV5, HPV8, and HPV14.[88]
Cause
[edit]Transmission
[edit]Sexually transmitted HPV is divided into two categories: low-risk and high-risk. Low-risk HPVs cause warts on or around the genitals. Type 6 and 11 cause 90% of all genital warts and recurrent respiratory papillomatosis that causes benign tumors in the air passages. High-risk HPVs cause cancer and consist of about twelve identified types.[11] Types 16 and 18 are responsible for causing most of HPV-caused cancers. These high-risk HPVs cause 5% of the cancers in the world. In the United States, high-risk HPVs cause 3% of all cancer cases in women and 2% in men.[89]
Risk factors for persistent genital HPV infections, which increase the risk of developing cancer, include early age of first sexual intercourse, multiple partners, smoking, and immunosuppression.[1] Genital HPV is spread by sustained direct skin-to-skin contact, with vaginal, anal, and oral sex being the most common methods.[4][22] Occasionally, it can spread from manual sex or from a mother to her baby during pregnancy.[90][91] HPV is difficult to remove via standard hospital disinfection techniques and may be transmitted in a healthcare setting on re-usable gynecological equipment, such as vaginal ultrasound transducers. The period of communicability is still unknown, but probably at least as long as visible HPV lesions persist. HPV may still be transmitted even after lesions are treated and no longer visible or present.[92]
Perinatal
[edit]Although genital HPV types can be transmitted from mother to child during birth, the appearance of genital HPV-related diseases in newborns is rare. However, the lack of appearance does not rule out asymptomatic latent infection, as the virus has proven to be capable of hiding for decades. Perinatal transmission of HPV types 6 and 11 can result in the development of juvenile-onset recurrent respiratory papillomatosis (JORRP). JORRP is very rare, with rates of about 2 cases per 100,000 children in the United States.[40] Although JORRP rates are substantially higher if a woman presents with genital warts at the time of giving birth, the risk of JORRP in such cases is still less than 1%.[citation needed]
Genital infections
[edit]Genital HPV infections are transmitted primarily by contact with the genitals, anus, or mouth of an infected sexual partner.[93]
Of the 120 known human papillomaviruses, 51 species and three subtypes infect the genital mucosa.[94] Fifteen are classified as high-risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82), three as probable high-risk (26, 53, and 66), and twelve as low-risk (6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, and 89).[21]
Condoms do not completely protect from the virus because the areas around the genitals including the inner thigh area are not covered, thus exposing these areas to the infected person's skin.[95]
Hands
[edit]Studies have shown HPV transmission between the hands and genitals of the same person and sexual partners. Hernandez tested the genitals and dominant hand of each person in 25 heterosexual couples every other month for an average of seven months. She found two couples where the man's genitals infected the woman's hand with high-risk HPV, two where her hand infected his genitals, one where her genitals infected his hand, two each where he infected his own hand, and she infected her own hand.[96] Hands were not the main source of transmission in these 25 couples, but they were significant.[citation needed]
Partridge reports men's fingertips became positive for high-risk HPV at more than half the rate (26% per two years) as their genitals (48%).[97] Winer reports 14% of fingertip samples from sexually active women were positive.[98]
Non-sexual hand contact seems to have little or no role in HPV transmission. Winer found all fourteen fingertip samples from virgin women negative at the start of her fingertip study.[98] In a separate report on genital HPV infection, 1% of virgin women (1 of 76) with no sexual contact tested positive for HPV, while 10% of virgin women reporting non-penetrative sexual contact were positive (7 of 72).[99]
Shared objects
[edit]Sharing of possibly contaminated objects, for example, razors,[92] may transmit HPV.[100][101][102] Although possible, transmission by routes other than sexual intercourse is less common for female genital HPV infection.[93] Fingers-genital contact is a possible way of transmission but unlikely to be a significant source.[98][103]
Blood
[edit]Though it has traditionally been assumed that HPV is not transmissible via blood – as it is thought to only infect cutaneous and mucosal tissues – recent studies have called this notion into question. Historically, HPV DNA has been detected in the blood of cervical cancer patients.[104] In 2005, a group reported that, in frozen blood samples of 57 sexually naive pediatric patients who had vertical or transfusion-acquired HIV infection, 8 (14.0%) of these samples also tested positive for HPV-16.[105] This seems to indicate that it may be possible for HPV to be transmitted via blood transfusion. However, as non-sexual transmission of HPV by other means is not uncommon, this could not be definitively proven. In 2009, a group tested Australian Red Cross blood samples from 180 healthy male donors for HPV, and subsequently found DNA of one or more strains of the virus in 15 (8.3%) of the samples.[106] However, it is important to note that detecting the presence of HPV DNA in blood is not the same as detecting the virus itself in blood, and whether or not the virus itself can or does reside in blood in infected individuals is still unknown. As such, it remains to be determined whether HPV can or cannot be transmitted via blood.[104] This is of concern, as blood donations are not currently screened for HPV, and at least some organizations such as the American Red Cross and other Red Cross societies do not presently appear to disallow HPV-positive individuals from donating blood.[107]
Surgery
[edit]Hospital transmission of HPV, especially to surgical staff, has been documented. Surgeons, including urologists and/or anyone in the room, are subject to HPV infection by inhalation of noxious viral particles during electrocautery or laser ablation of a condyloma (wart).[108] There has been a case report of a laser surgeon who developed extensive laryngeal papillomatosis after providing laser ablation to patients with anogenital condylomata.[108]
Virology
[edit]
HPV infection is limited to the basal cells of stratified epithelium, the only tissue in which they replicate.[110] The virus cannot bind to live tissue; instead, it infects epithelial tissues through micro-abrasions or other epithelial trauma that exposes segments of the basement membrane.[110] The infectious process is slow, taking 12–24 hours for initiation of transcription. It is believed that involved antibodies play a major neutralizing role while the virions still reside on the basement membrane and cell surfaces.[110]
HPV lesions are thought to arise from the proliferation of infected basal keratinocytes. Infection typically occurs when basal cells in the host are exposed to the infectious virus through a disturbed epithelial barrier as would occur during sexual intercourse or after minor skin abrasions. HPV infections have not been shown to be cytolytic; rather, viral particles are released as a result of degeneration of desquamating cells. HPV can survive for many months and at low temperatures without a host; therefore, an individual with plantar warts can spread the virus by walking barefoot.[38]
HPV is a small double-stranded circular DNA virus with a genome of approximately 8000 base pairs.[22][111] The HPV life cycle strictly follows the differentiation program of the host keratinocyte. It is thought that the HPV virion infects epithelial tissues through micro-abrasions, whereby the virion associates with putative receptors such as alpha integrins, laminins, and annexin A2[112] leading to the entry of the virions into basal epithelial cells through clathrin-mediated endocytosis and/or caveolin-mediated endocytosis depending on the type of HPV.[113] At this point, the viral genome is transported to the nucleus by unknown mechanisms and establishes itself at a copy number of 10-200 viral genomes per cell. A sophisticated transcriptional cascade then occurs as the host keratinocyte begins to divide and become increasingly differentiated in the upper layers of the epithelium.[citation needed]
Evolution
[edit]The phylogeny of the various strains of HPV generally reflects the migration patterns of Homo sapiens and suggests that HPV may have diversified along with the human population. Studies suggest that HPV evolved along five major branches that reflect the ethnicity of human hosts, and diversified along with the human population.[114]
Researchers initially identified two major variants of HPV16, European (HPV16-E), and Non-European (HPV16-NE).[115] More recent analyses based on thousands of HPV16 genomes show that indeed two major clades exist, that are further subdivided into four lineages (designated A-D) and even further subdivided into 16 sublineages (A1–4, B1–4, C1–4 and D1–4).[116][117] The A1-A3 sublineages constitute the European variant, A4 the Asian variant, B1-B4 the African type I variant, C1–C4 the African type II variant, D1 the North American variant, D2 the Asian American type I variant, D3 the Asian American type II variant.[116] The various lineages and sublineages have different oncogenic capacity, where overall, the non-European lineages are considered to increase the risk for cancer.[118] Although HPV16 is a DNA virus, there are signs of recombination among the different lineages.[117][119] Based on an analysis of more than 3600 genomes, between 0.3 and 1.2% of them could be recombinant.[117] Thus, ideally, genotyping (for cancer-risk assessment) of HPV16 should not be based only on certain genes, but on all genes from the entire genome.[117]
A bioinformatics tool named HPV16-Genotyper performs i) HPV16 lineage genotyping, ii) detects potential recombination events, iii) identifies, within the submitted sequences, mutations/SNPs that have been reported (in literature) to increase the risk for cancer.[117]
E6/E7 proteins
[edit]
The two primary oncoproteins of high-risk HPV types are E6 and E7. The "E" designation indicates that these two proteins are early proteins (expressed early in the HPV life cycle), while the "L" designation indicates that they are late proteins (late expression).[55] The HPV genome is composed of six early (E1, E2, E4, E5, E6, and E7) open reading frames (ORF), two late (L1 and L2) ORFs, and a non-coding long control region (LCR).[121] After the host cell is infected viral early promoter is activated and a polycistronic primary RNA containing all six early ORFs is transcribed. This polycistronic RNA then undergoes active RNA splicing to generate multiple isoforms of mRNAs.[122] One of the spliced isoform RNAs, E6*I, serves as an E7 mRNA to translate E7 protein.[123] However, viral early transcription subjects to viral E2 regulation and high E2 levels repress the transcription. HPV genomes integrate into the host genome by disruption of E2 ORF, preventing E2 repression on E6 and E7. Thus, viral genome integration into the host DNA genome increases E6 and E7 expression to promote cellular proliferation and the chance of malignancy. The degree to which E6 and E7 are expressed is correlated with the type of cervical lesion that can ultimately develop.[111]
- Role in cancer
Sometimes papillomavirus genomes are found integrated into the host genome, and this is especially noticeable with oncogenic HPVs.[124] The E6/E7 proteins inactivate two tumor suppressor proteins, p53 (inactivated by E6) and pRb (inactivated by E7).[125] The viral oncogenes E6 and E7[126] are thought to modify the cell cycle so as to retain the differentiating host keratinocyte in a state that is favourable to the amplification of viral genome replication and consequent late gene expression. E6 in association with host E6-associated protein, which has ubiquitin ligase activity, acts to ubiquitinate p53, leading to its proteosomal degradation. E7 (in oncogenic HPVs) acts as the primary transforming protein. E7 competes for retinoblastoma protein (pRb) binding, freeing the transcription factor E2F to transactivate its targets, thus pushing the cell cycle forward. All HPV can induce transient proliferation, but only strains 16 and 18 can immortalize cell lines in vitro. It has also been shown that HPV 16 and 18 cannot immortalize primary rat cells alone; there needs to be activation of the ras oncogene. In the upper layers of the host epithelium, the late genes L1 and L2 are transcribed/translated and serve as structural proteins that encapsidate the amplified viral genomes. Once the genome is encapsidated, the capsid appears to undergo a redox-dependent assembly/maturation event, which is tied to a natural redox gradient that spans both suprabasal and cornified epithelial tissue layers. This assembly/maturation event stabilizes virions and increases their specific infectivity.[127] Virions can then be sloughed off in the dead squames of the host epithelium and the viral lifecycle continues.[128] A 2010 study has found that E6 and E7 are involved in beta-catenin nuclear accumulation and activation of Wnt signaling in HPV-induced cancers.[129]
Latency period
[edit]Once an HPV virion invades a cell, an active infection occurs, and the virus can be transmitted. Several months to years may elapse before squamous intraepithelial lesions (SIL) develop and can be clinically detected. The time from active infection to clinically detectable disease may make it difficult for epidemiologists to establish which partner was the source of infection.[108]
Clearance
[edit]Most HPV infections are cleared up by most people without medical action or consequences. The table provides data for high-risk types (i.e. the types found in cancers).[citation needed]
| Months after initial positive test | 8 months | 12 months | 18 months |
|---|---|---|---|
| % of men tested negative | 70% | 80% | 100% |
Clearing an infection does not always create immunity if there is a new or continuing source of infection. Hernandez' 2005-6 study of 25 couples reports "A number of instances indicated apparent reinfection [from partner] after viral clearance."[96]
Diagnosis
[edit]
Over 200 types of HPV have been identified, and they are designated by numbers.[11][8][125] They may be divided into "low-risk" and "high-risk" types. Low-risk types cause warts and high-risk types can cause lesions or cancer.[132][133]
Cervical testing
[edit]Guidelines from the American Cancer Society recommend different screening strategies for cervical cancer based on a woman's age, screening history, risk factors, and choice of tests.[134] Because of the link between HPV and cervical cancer, the ACS currently recommends early detection of cervical cancer in average-risk asymptomatic adults primarily with cervical cytology by Pap smear, regardless of HPV vaccination status. Women aged 30–65 should preferably be tested every 5 years with both the HPV test and the Pap test. In other age groups, a Pap test alone can suffice unless they have been diagnosed with atypical squamous cells of undetermined significance (ASC-US).[135] Co-testing with a Pap test and HPV test is recommended because it decreases the rate of false-negatives. According to the National Cancer Institute, "The most common test detects DNA from several high-risk HPV types, but it cannot identify the types that are present. Another test is specific for DNA from HPV types 16 and 18, the two types that cause most HPV-associated cancers. A third test can detect DNA from several high-risk HPV types and can indicate whether HPV-16 or HPV-18 is present. A fourth test detects RNA from the most common high-risk HPV types. These tests can detect HPV infections before cell abnormalities are evident.[citation needed]
"Theoretically, the HPV DNA and RNA tests could be used to identify HPV infections in cells taken from any part of the body. However, the tests are approved by the FDA for only two indications: for follow-up testing of women who seem to have abnormal Pap test results and for cervical cancer screening in combination with a Pap test among women over age 30."[136]
Mouth testing
[edit]Guidelines for oropharyngeal cancer screening by the Preventive Services Task Force and American Dental Association in the U.S. suggest conventional visual examination, but because some parts of the oropharynx are hard to see, this cancer is often only detected in later stages.[67]
The diagnosis of oropharyngeal cancer occurs by biopsy of exfoliated cells or tissues. The National Comprehensive Cancer Network and College of American Pathologists recommend testing for HPV in oropharyngeal cancer.[67] However, while testing is recommended, there is no specific type of test used to detect HPV from oral tumors that is currently recommended by the FDA in the United States. Because HPV type 16 is the most common type found in oropharyngeal cancer, p16 immunohistochemistry is one test option used to determine if HPV is present,[137] which can help determine course of treatment since tumors that are negative for p16 have better outcomes. Another option that has emerged as a reliable option is HPV DNA in situ hybridization (ISH) which allows for visualization of the HPV.[67]
Testing men
[edit]There is not a wide range of tests available even though HPV is common; most studies of HPV used tools and custom analysis not available to the general public.[138][needs update] Clinicians often depend on the vaccine among young people and high clearance rates (see Clearance subsection in Virology) to create a low risk of disease and mortality, and treat the cancers when they appear. Others believe that reducing HPV infection in more men and women, even when it has no symptoms, is important (herd immunity) to prevent more cancers rather than just treating them.[139][140][needs update] Where tests are used, negative test results show safety from transmission, and positive test results show where shielding (condoms, gloves) is needed to prevent transmission until the infection clears.[141]
Studies have tested for and found HPV in men, including high-risk types (i.e. the types found in cancers), on fingers, mouth, saliva, anus, urethra, urine, semen, blood, scrotum and penis.[138]
The aforementioned Qiagen/Digene kit was successfully used off-label to test the penis, scrotum, and anus[142] of men in long-term relationships with women who were positive for high-risk HPV. Of these men, 60% were found to carry the virus, primarily on the penis.[142][needs update] Similar studies have been conducted on women using cytobrushes - an endocervical brush for sampling the cervix in females - and custom analysis.[143][144][needs update]
In one study researchers sampled subjects' urethra, scrotum, and penis.[143][144][needs update] Samples taken from the urethra added less than 1% to the HPV rate. Studies like this led Giuliano to recommend sampling the glans, shaft, and crease between them, along with the scrotum, since sampling the urethra or anus added very little to the diagnosis.[97] Dunne recommends the glans, shaft, their crease, and the foreskin.[138]
In one study the subjects were asked not to wash their genitals for 12 hours before sampling, including the urethra as well as the scrotum and the penis.[143] Other studies are silent on washing – a particular gap in studies of the hands.[citation needed]
One small study used wet cytobrushes, rather than wet the skin.[144] It found a higher proportion of men to be HPV-positive when the skin was rubbed with a 600 grit emery paper before being swabbed with the brush, rather than swabbed with no preparation. It's unclear whether the emery paper collected the virions or simply loosened them for the swab to collect.[citation needed]
Studies have found self-collection (with emery paper and Dacron swabs) as effective as collection done by a clinician, and sometimes more so, since patients were more willing than a clinician to scrape vigorously.[145][needs update][146] Women had similar success in self-sampling using tampons, swabs, cytobrushes, and lavage.[147][needs update]
Several studies used cytobrushes to sample fingertips and under fingernails, without wetting the area or the brush.[98][103][148][needs update]
Other studies analyzed urine, semen, and blood and found varying amounts of HPV,[138] but there is not a publicly available test for those yet.
Other testing
[edit]Although it is possible to test for HPV DNA in other kinds of infections,[138] there are no FDA-approved tests for general screening in the United States[149] or tests approved by the Canadian government,[150] since the testing is inconclusive and considered medically unnecessary.[151]
Genital warts are the only visible sign of low-risk genital HPV and can be identified with a visual check. These visible growths, however, are the result of non-carcinogenic HPV types. Five percent acetic acid (vinegar) is used to identify both warts and squamous intraepithelial neoplasia (SIL) lesions with limited success[citation needed] by causing abnormal tissue to appear white, but most doctors have found this technique helpful only in moist areas, such as the female genital tract.[citation needed] At this time, HPV tests for males are used only in research.[citation needed]
Research into testing for HPV by antibody presence has been done. The approach is looking for an immune response in blood, which would contain antibodies for HPV if the patient is HPV positive.[152][153][154][155] The reliability of such tests has not been proven, as there has not been a FDA approved product as of August 2018;[156] testing by blood would be a less invasive test for screening purposes.
Prevention
[edit]The HPV vaccines can prevent the most common types of infection.[4] To be effective they must be used before an infection occurs and are therefore recommended between the ages of nine and thirteen. Cervical cancer screening, such as with the Papanicolaou test (pap) or looking at the cervix after using acetic acid, can detect early cancer or abnormal cells that may develop into cancer. This allows for early treatment which results in better outcomes.[1] Screening has reduced both the number and deaths from cervical cancer in the developed world.[18] Warts can be removed by freezing.[5]
Vaccines
[edit]Three vaccines are available to prevent infection by some HPV types: Gardasil, Gardasil 9 and Cervarix; all three protect against initial infection with HPV types 16 and 18, which cause most of the HPV-associated cancer cases. Gardasil also protects against HPV types 6 and 11, which cause 90% of genital warts. Gardasil is a recombinant quadrivalent vaccine, whereas Cervarix is bivalent, and is prepared from virus-like particles (VLP) of the L1 capsid protein. Gardasil 9 is nonavalent, having the potential to prevent about 90% of cervical, vulvar, vaginal, and anal cancers. It can protect for HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58; the latter five cause up to 20% of cervical cancers which were not previously covered.[157]
The vaccines provide little benefit to women already infected with HPV types 16 and 18.[158] For this reason, the vaccine is recommended primarily for those women not yet having been exposed to HPV during sex. The World Health Organization position paper on HPV vaccination clearly outlines appropriate, cost-effective strategies for using HPV vaccine in public sector programs.[159]
There is high-certainty evidence that HPV vaccines protect against precancerous cervical lesions in young women, particularly those vaccinated aged 15 to 26.[160] HPV vaccines do not increase the risk of serious adverse events.[160] Longer follow-up is needed to monitor the impact of HPV vaccines on cervical cancer.[160]
The CDC recommends the vaccines be delivered in two shots at an interval of at least 6 months for those aged 11–12, and three doses for those 13 and older.[161] In most countries, they are funded only for female use, but are approved for male use in many countries, and funded for teenage boys in Australia. The vaccine does not have any therapeutic effect on existing HPV infections or cervical lesions.[162] In 2010, 49% of teenage girls in the US got the HPV vaccine.[citation needed]
Following studies suggesting that the vaccine is more effective in younger girls[163] than in older teenagers, the United Kingdom, Switzerland, Mexico, the Netherlands, and Quebec began offering the vaccine in a two-dose schedule for girls aged under 15 in 2014.[citation needed]
Cervical cancer screening recommendations have not changed for females who receive the HPV vaccine. It remains a recommendation that women continue cervical screening, such as Pap smear testing, even after receiving the vaccine, since it does not prevent all types of cervical cancer.[162][164]
Both men and women are carriers of HPV.[165] The Gardasil vaccine also protects men against anal cancers and warts and genital warts.[166]
Duration of both vaccines' efficacy has been observed since they were first developed, and is expected to be long-lasting.[167]
In December 2014, the FDA approved a nine-valent Gardasil-based vaccine, Gardasil 9, to protect against infection with the four strains of HPV covered by the first generation of Gardasil as well as five other strains responsible for 20% of cervical cancers (HPV-31, HPV-33, HPV-45, HPV-52, and HPV-58).[168]
Condoms
[edit]The Centers for Disease Control and Prevention says that male "condom use may reduce the risk for genital human papillomavirus (HPV) infection" but provides a lesser degree of protection compared with other sexual transmitted infections "because HPV also may be transmitted by exposure to areas (e.g., infected skin or mucosal surfaces) that are not covered or protected by the condom."[169]
Disinfection
[edit]The virus is unusually hardy and is immune to most common disinfectants. It is the first virus ever shown to be resistant to inactivation by glutaraldehyde, which is among the most common strong disinfectants used in hospitals.[170] Diluted sodium hypochlorite bleach is effective,[170] but cannot be used on some types of re-usable equipment, such as ultrasound transducers.[90] As a result of these difficulties, there is developing concern about the possibility of transmitting the virus on healthcare equipment, particularly reusable gynecological equipment that cannot be autoclaved.[171][172] For such equipment, some health authorities encourage use of UV disinfection[173] or a non-hypochlorite "oxidizing‐based high‐level disinfectant [bleach] with label claims for non‐enveloped viruses",[174] such as a strong hydrogen peroxide solution[175][173] or chlorine dioxide wipes.[173] Such disinfection methods are expected to be relatively effective against HPV.[citation needed]
Management
[edit]There is currently no specific treatment for HPV infection.[176][177][178] However, the viral infection is usually cleared to undetectable levels by the immune system.[179] According to the Centers for Disease Control and Prevention, the body's immune system clears HPV naturally within two years for 90% of cases (see Clearance subsection in Virology for more detail).[176] However, experts do not agree on whether the virus is eliminated or reduced to undetectable levels, and it is difficult to know when it is contagious.[180][needs update]
Follow up care is usually recommended and practiced by many health clinics.[181] Follow-up is sometimes not successful because a portion of those treated do not return to be evaluated. In addition to the normal methods of phone calls and mail, text messaging and email can improve the number of people who return for care.[182] As of 2015 it is unclear the best method of follow up following treatment of cervical intraepithelial neoplasia.[183]
Epidemiology
[edit]Globally, 12% of women are positive for HPV DNA, with rates varying by age and country.[184] The highest rates of HPV are in younger women, with a rate of 24% in women under 25 years.[185] Rates decline in older age groups in Europe and the Americas, but less so in Africa and Asia. The rates are highest in Sub-Saharan Africa (24%) and Eastern Europe (21%) and lowest in North America (5%) and Western Asia (2%).[184]
The most common types of HPV worldwide are HPV16 (3.2%), HPV18 (1.4%), HPV52 (0.9%), HPV31 (0.8%), and HPV58 (0.7%). High-risk types of HPV are also distributed unevenly, with HPV16 having a rate of around 13% in Africa and 30% in West and Central Asia.[185]
Like many diseases, HPV disproportionately affects low-income and resource-poor countries. The higher rates of HPV in Sub-Saharan Africa, for example, may be related to high exposure to human immunodeficiency virus (HIV) in the region. Other factors that impact the global spread of disease are sexual behaviors including age of sexual debut, number of sexual partners, and ease of access to barrier contraception, all of which vary globally.[184][186]
The papilloma virus is not only widespread among women, but is also behind most cases of oropharyngeal cancer, which is the fastest growing cancer among young adults in Western countries.[187] Moreover, as of 2025, papilloma virus is the most prevalent sexually transmitted infection in the world.[187]
United States
[edit]| Age (years) | Prevalence (%) |
|---|---|
| 14 to 19 | 24.5% |
| 20 to 24 | 44.8% |
| 25 to 29 | 27.4% |
| 30 to 39 | 27.5% |
| 40 to 49 | 25.2% |
| 50 to 59 | 19.6% |
| 14 to 59 | 26.8% |
HPV is estimated to be the most common sexually transmitted infection in the United States.[188] Most sexually active men and women will probably acquire genital HPV infection at some point in their lives.[24] The American Social Health Association estimates that about 75–80% of sexually active Americans will be infected with HPV at some point in their lifetime.[189][190] By the age of 50 more than 80% of American women will have contracted at least one strain of genital HPV.[188][191] It was estimated that, in the year 2000, there were approximately 6.2 million new HPV infections among Americans aged 15–44; of these, an estimated 74% occurred to people between ages of 15 and 24.[192] Of the STIs studied, genital HPV was the most commonly acquired.[192] In the United States, it is estimated that 10% of the population has an active HPV infection, 4% has an infection that has caused cytological abnormalities, and an additional 1% has an infection causing genital warts.[193]
Estimates of HPV prevalence vary from 14% to more than 90%.[194] One reason for the difference is that some studies report women who currently have a detectable infection, while other studies report women who have ever had a detectable infection.[195][196] Another cause of discrepancy is the difference in strains that were tested for.[citation needed]
One study found that, during 2003–2004, at any given time, 26.8% of women aged 14 to 59 were infected with at least one type of HPV. This was higher than previous estimates; 15.2% were infected with one or more of the high-risk types that can cause cancer.[188][197]
The prevalence for high-risk and low-risk types is roughly similar over time.[188]
Human papillomavirus is not included among the diseases that are typically reportable to the CDC as of 2011.[198][199]
Ireland
[edit]On average 538 cases of HPV-associated cancers were diagnosed per year in Ireland during the period 2010 to 2014.[200] Cervical cancer was the most frequent HPV-associated cancer with on average 292 cases per year (74% of the female total, and 54% of the overall total of HPV-associated cancers).[200] A study of 996 cervical cytology samples in an Irish urban female, opportunistically screened population, found an overall HPV prevalence of 19.8%, HPV 16 at 20% and HPV 18 at 12% were the commonest high-risk types detected. In Europe, types 16 and 18 are responsible for over 70% of cervical cancers.[201] Overall rates of HPV-associated invasive cancers may be increasing. Between 1994 and 2014, there was a 2% increase in the rate of HPV-associated invasive cancers per year for both sexes in Ireland.[200]
As HPV is known to be associated with anogenital warts, these are notifiable to the Health Protection Surveillance Centre (HPSC). Genital warts are the second most common STI in Ireland.[202] There were 1,281 cases of anogenital warts notified in 2017, which was a decrease on the 2016 figure of 1,593.[203] The highest age-specific rate for both male and female was in the 25–29 year old age range; 53% of cases were among males.[203]
Sri Lanka
[edit]In Sri Lanka, the prevalence of HPV is 15.5% regardless of cytological abnormalities.[204]
Inner Mongolia
[edit]In the Autonomous Region of Inner Mongolia overall HPV prevalence is 14.5% but shows substantial ethnical disparity, the prevalence in Mongolian women (14.9%) being much higher than that of Han participants (4.3%).[205] Urbanization, the number of sex partners, and PAP history appear as risk factors for HPV infection in Han, but not in Mongolian women. The region is thus an important example that the epidemiology of HPV is more related to cultural and ethnical factors and not to geography per se.[citation needed]
History
[edit]One of the first studies linking risk of uterine carcinoma with the number of sexual activity was performed in 1842, in Verona. Dr. Domenico Rigoni-Stern observed that uterine cancer incidence among Catholic nuns living in convents in the countryside was lower than in women living in the city. Highest incidence was seen for prostitutes, thereby linking uterine cancer prevalence to the number of sexual partners, and suggesting that this disease might have a sexually transmissible component.[206]
In 1972, the association of the human papillomaviruses with skin cancer in epidermodysplasia verruciformis was proposed by Stefania Jabłońska in Poland. In 1976 Harald zur Hausen published the hypothesis that human papillomavirus plays an important role in the cause of cervical cancer. In 1978, Jabłońska and Gerard Orth at the Pasteur Institute discovered HPV-5 in skin cancer.[207] In 1983 and 1984 zur Hausen and his collaborators identified HPV16 and HPV18 in cervical cancer.[208]
The HeLa cell line contains extra DNA in its genome that originated from HPV type 18.[209]
Research
[edit]The Ludwig-McGill HPV Cohort is one of the world's largest longitudinal studies of the natural history of human papillomavirus (HPV) infection and cervical cancer risk. It was established in 1993 by Ludwig Cancer Research and McGill University in Montreal, Canada.[210]
See also
[edit]References
[edit]- ^ a b c d e f g h i j k l m n o p q r s t "Human papillomavirus (HPV) and cervical cancer - WHO". World Health Organization. 22 February 2022. Archived from the original on 22 April 2023.
- ^ a b c d e Ljubojevic S, Skerlev M (2014). "HPV-associated diseases". Clinics in Dermatology. 32 (2): 227–34. doi:10.1016/j.clindermatol.2013.08.007. PMID 24559558.
- ^ a b c d e f Anjum, Fatima; Zohaib, Jamal (4 December 2020). "Oropharyngeal Squamous Cell Carcinoma". Definitions (Updated ed.). Treasure Island (FL): StatPearls Publishing. doi:10.32388/G6TG1L. PMID 33085415. S2CID 229252540. Bookshelf ID: NBK563268. Retrieved 6 February 2021 – via NCBI.
- ^ a b c d e f g h i j "What is HPV?". Centers for Disease Control and Prevention. 28 December 2015. Archived from the original on 7 August 2016. Retrieved 10 August 2016.
- ^ a b c d e f Milner DA (2015). Diagnostic Pathology: Infectious Diseases. Elsevier Health Sciences. p. 40. ISBN 978-0-323-40037-4. Archived from the original on 11 September 2017.
- ^ "Fact Sheet for Public Health Personnel | Condom Effectiveness | CDC". Centers for Disease Control and Prevention. 25 March 2013. Archived from the original on 27 May 2017. Retrieved 1 May 2017.
- ^ a b c "The Link Between HPV and Cancer". Centers for Disease Control and Prevention. 30 September 2015. Archived from the original on 9 November 2015. Retrieved 11 August 2016.
- ^ a b Bzhalava D, Guan P, Franceschi S, Dillner J, Clifford G (October 2013). "A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types". Virology. 445 (1–2): 224–31. doi:10.1016/j.virol.2013.07.015. PMID 23928291.
- ^ Lange, S.; Son, S.; Jensen, M.; Medenblik, A.; Sullivan, J.; Basting, E.; Stuart, G. (2024). "HPV (Human Papillomavirus)". Encyclopedia of Sexual Psychology and Behavior. Springer, Cham. pp. 1–2. doi:10.1007/978-3-031-08956-5_1137-1. ISBN 978-3-031-08956-5.
- ^ "HPV reference clones – International Human Papillomavirus Reference Center". Retrieved 11 March 2025.
- ^ a b c d e f g h "HPV and Cancer - National Cancer Institute". National Cancer Institute. 18 October 2023. Retrieved 18 January 2024.
- ^ a b c "Human Papillomavirus (HPV) Questions and Answers". Centers for Disease Control and Prevention. 28 December 2015. Archived from the original on 11 August 2016. Retrieved 11 August 2016.
- ^ "Pink Book (Human Papillomavirus)" (PDF). Centers for Disease Control and Prevention. Archived (PDF) from the original on 21 March 2017. Retrieved 18 April 2017.
- ^ "5 Things You Might Not Know About Human Papillomavirus". Centers for Disease Control and Prevention. 20 January 2016. Retrieved 22 May 2020.
- ^ "Human Papilloma Virus (HPV)" (PDF). WRHA. 18 November 2019. Retrieved 26 March 2019.
- ^ Meyers J, Ryndock E, Conway MJ, Meyers C, Robison R (June 2014). "Susceptibility of high-risk human papillomavirus type 16 to clinical disinfectants". J Antimicrob Chemother. 69 (6): 1546–50. doi:10.1093/jac/dku006. PMC 4019329. PMID 24500190.
- ^ Ellingson, Mallory K.; Sheikha, Hassan; Nyhan, Kate; Oliveira, Carlos R.; Niccolai, Linda M. (August 2023). "Human papillomavirus vaccine effectiveness by age at vaccination: A systematic review". Human Vaccines & Immunotherapeutics. 19 (2). doi:10.1080/21645515.2023.2239085. ISSN 2164-5515. PMC 10399474. Archived from the original on 22 February 2025.
- ^ a b Sawaya GF, Kulasingam S, Denberg TD, Qaseem A (June 2015). "Cervical Cancer Screening in Average-Risk Women: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians". Annals of Internal Medicine. 162 (12): 851–9. doi:10.7326/M14-2426. PMID 25928075. S2CID 25957804.
- ^ a b c Tyring S, Moore AY, Lupi O (2016). Mucocutaneous Manifestations of Viral Diseases: An Illustrated Guide to Diagnosis and Management (2nd ed.). CRC Press. p. 207. ISBN 978-1-4200-7313-3.
- ^ M Al Aboud A, Nigam PK (2022). "Wart (Plantar, Verruca Vulgaris, Verrucae)". Stat Pearls. PMID 28613701. Retrieved 4 December 2019.
- ^ a b Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. (International Agency for Research on Cancer Multicenter Cervical Cancer Study Group) (February 2003). "Epidemiologic classification of human papillomavirus types associated with cervical cancer". The New England Journal of Medicine. 348 (6): 518–27. doi:10.1056/NEJMoa021641. hdl:2445/122831. PMID 12571259. S2CID 1451343.
- ^ a b c Pahud BA, Ault KA (December 2015). "The Expanded Impact of Human Papillomavirus Vaccine". Infectious Disease Clinics of North America (Review). 29 (4): 715–24. doi:10.1016/j.idc.2015.07.007. PMID 26610422.
- ^ Nowińska K, Ciesielska U, Podhorska-Okołów M, Dzięgiel P (2017). "The role of human papillomavirus in oncogenic transformation and its contribution to the etiology of precancerous lesions and cancer of the larynx: A review". Advances in Clinical and Experimental Medicine. 26 (3): 539–547. doi:10.17219/acem/67461. PMID 28791831.
- ^ a b c Baseman JG, Koutsky LA (March 2005). "The epidemiology of human papillomavirus infections". Journal of Clinical Virology. 32 (Suppl 1): S16-24. doi:10.1016/j.jcv.2004.12.008. PMID 15753008.
Overall, these DNA-based studies, combined with measurements of type-specific antibodies against HPV capsid antigens, have shown that most (>50%) sexually active women have been infected by one or more genital HPV types at some point in time [S17].
- ^ Noel J, Lespagnard L, Fayt I, Verhest A, Dargent J (January 2001). "Evidence of human papilloma virus infection but lack of Epstein-Barr virus in lymphoepithelioma-like carcinoma of uterine cervix: report of two cases and review of the literature". Human Pathology. 32 (1): 135–8. doi:10.1053/hupa.2001.20901. PMID 11172309.
- ^ "Vulvar Intraepithelial Neoplasia: Varied signs, varied symptoms: what you need to know". www.advanceweb.com. Archived from the original on 16 July 2012. Retrieved 5 August 2009.
- ^ a b c Kumar V, Abbas AK, Fausto N, Mitchell R (2007). "Chapter 19 The Female Genital System and Breast". Robbins Basic Pathology (8 ed.). Philadelphia: Saunders. ISBN 978-1-4160-2973-1.
- ^ Palefsky JM, Holly EA, Ralston ML, Jay N (February 1998). "Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men". The Journal of Infectious Diseases. 177 (2): 361–7. doi:10.1086/514194. PMID 9466522.
- ^ Muñoz N, Castellsagué X, de González AB, Gissmann L (August 2006). "Chapter 1: HPV in the etiology of human cancer". Vaccine. 24 Suppl 3 (3): S3/1–10. doi:10.1016/j.vaccine.2006.05.115. PMID 16949995.
- ^ a b c World Health Organization (December 2022). "Human papillomavirus vaccines: WHO position paper (2022 update)". Weekly Epidemiological Record. 97 (50): 645–672. hdl:10665/365351.
- ^ Antonsson A, Forslund O, Ekberg H, Sterner G, Hansson BG (December 2000). "The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses". Journal of Virology. 74 (24): 11636–41. doi:10.1128/JVI.74.24.11636-11641.2000. PMC 112445. PMID 11090162.
- ^ Mayo Clinic.com, Common warts, http://www.mayoclinic.com/print/common-warts/DS00370/ Archived 17 October 2011 at the Wayback Machine
- ^ Al Aboud, Ahmad M.; Nigam, Pramod K. (11 August 2020). "Wart". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 28613701.
- ^ Lountzis NI, Rahman O (July 2008). "Images in clinical medicine. Digital verrucae". The New England Journal of Medicine. 359 (2): 177. doi:10.1056/NEJMicm071912. PMID 18614785.
- ^ MedlinePlus, Warts, https://www.nlm.nih.gov/medlineplus/warts.html#cat42 Archived 5 June 2016 at the Wayback Machine (general reference with links). Also, see
- ^ a b World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 5.12. ISBN 978-92-832-0429-9.
- ^ Greer CE, Wheeler CM, Ladner MB, Beutner K, Coyne MY, Liang H, et al. (August 1995). "Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts". Journal of Clinical Microbiology. 33 (8): 2058–63. doi:10.1128/jcm.33.8.2058-2063.1995. PMC 228335. PMID 7559948.
- ^ a b "Human Papillomavirus". Medscape. 16 October 2018. Archived from the original on 29 November 2016.
- ^ "Photos of larynx Papillomas — Voice Medicine, New York". Voicemedicine.com. Archived from the original on 12 June 2010. Retrieved 29 August 2010.
- ^ a b c d Sinal SH, Woods CR (October 2005). "Human papillomavirus infections of the genital and respiratory tracts in young children". Seminars in Pediatric Infectious Diseases. 16 (4): 306–16. doi:10.1053/j.spid.2005.06.010. PMID 16210110.
- ^ Wu R, Sun S, Steinberg BM (2003). "Requirement of STAT3 activation for differentiation of mucosal stratified squamous epithelium". Molecular Medicine. 9 (3–4): 77–84. doi:10.2119/2003-00001.Wu. PMC 1430729. PMID 12865943.
- ^ Moore CE, Wiatrak BJ, McClatchey KD, Koopmann CF, Thomas GR, Bradford CR, Carey TE (May 1999). "High-risk human papillomavirus types and squamous cell carcinoma in patients with respiratory papillomas". Otolaryngology–Head and Neck Surgery. 120 (5): 698–705. doi:10.1053/hn.1999.v120.a91773. PMID 10229596. S2CID 6560398.
- ^ a b c Viens LJ, Henley SJ, Watson M, Markowitz LE, Thomas CC, Thompson TD, et al. (July 2016). "Human Papillomavirus-Associated Cancers – United States, 2008–2012". MMWR. Morbidity and Mortality Weekly Report. 65 (26): 661–6. doi:10.15585/mmwr.mm6526a1. PMID 27387669.
- ^ Schiffman M, Castle PE (November 2005). "The promise of global cervical-cancer prevention". The New England Journal of Medicine. 353 (20): 2101–4. doi:10.1056/NEJMp058171. PMID 16291978.
- ^ Alam S, Conway MJ, Chen HS, Meyers C (January 2008). "The cigarette smoke carcinogen benzo[a]pyrene enhances human papillomavirus synthesis". Journal of Virology. 82 (2): 1053–8. doi:10.1128/JVI.01813-07. PMC 2224590. PMID 17989183.
- ^ Lu B, Hagensee ME, Lee JH, Wu Y, Stockwell HG, Nielson CM, et al. (February 2010). "Epidemiologic factors associated with seropositivity to human papillomavirus type 16 and 18 virus-like particles and risk of subsequent infection in men". Cancer Epidemiology, Biomarkers & Prevention. 19 (2): 511–6. doi:10.1158/1055-9965.EPI-09-0790. PMID 20086109. S2CID 22440577.
- ^ Parfenov M, Pedamallu CS, Gehlenborg N, Freeman SS, Danilova L, Bristow CA, et al. (October 2014). "Characterization of HPV and host genome interactions in primary head and neck cancers". Proceedings of the National Academy of Sciences of the United States of America. 111 (43): 15544–9. Bibcode:2014PNAS..11115544P. doi:10.1073/pnas.1416074111. PMC 4217452. PMID 25313082.
- ^ Scheffner, Martin; Huibregtse, Jon M.; Vierstra, Richard D.; Howley, Peter M. (November 1993). "The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53". Cell. 75 (3): 495–505. doi:10.1016/0092-8674(93)90384-3. PMID 8221889. S2CID 27437768.
- ^ Zanier, Katia; Charbonnier, Sebastian; Sidi, Abdellahi Ould M'hamed Ould; McEwen, Alastair G.; Ferrario, Maria Giovanna; Poussin-Courmontagne, Pierre; Cura, Vincent; Brimer, Nicole; Babah, Khaled Ould; Ansari, Tina; Muller, Isabelle (8 February 2013). "Structural basis for hijacking of cellular LxxLL motifs by papillomavirus E6 oncoproteins". Science. 339 (6120): 694–698. Bibcode:2013Sci...339..694Z. doi:10.1126/science.1229934. ISSN 1095-9203. PMC 3899395. PMID 23393263.
- ^ Hafner, Antonina; Bulyk, Martha L.; Jambhekar, Ashwini; Lahav, Galit (April 2019). "The multiple mechanisms that regulate p53 activity and cell fate". Nature Reviews. Molecular Cell Biology. 20 (4): 199–210. doi:10.1038/s41580-019-0110-x. ISSN 1471-0080. PMID 30824861. S2CID 71143679.
- ^ Hanahan, Douglas; Weinberg, Robert A. (7 January 2000). "The Hallmarks of Cancer". Cell. 100 (1): 57–70. doi:10.1016/S0092-8674(00)81683-9. ISSN 0092-8674. PMID 10647931. S2CID 1478778.
- ^ Tungteakkhun, Sandy S.; Duerksen-Hughes, Penelope J. (2008). "Cellular binding partners of the human papillomavirus E6 protein". Archives of Virology. 153 (3): 397–408. doi:10.1007/s00705-007-0022-5. ISSN 0304-8608. PMC 2249614. PMID 18172569.
- ^ Karagas MR, Waterboer T, Li Z, Nelson HH, Michael KM, Bavinck JN, et al. (July 2010). "Genus beta human papillomaviruses and incidence of basal cell and squamous cell carcinomas of skin: population based case-control study". BMJ. 341: c2986. doi:10.1136/bmj.c2986. PMC 2900549. PMID 20616098.
- ^ Cohen J (April 2005). "Public health. High hopes and dilemmas for a cervical cancer vaccine". Science. 308 (5722): 618–21. doi:10.1126/science.308.5722.618. PMID 15860602. S2CID 31712160.
- ^ a b c Ault KA (2006). "Epidemiology and natural history of human papillomavirus infections in the female genital tract". Infectious Diseases in Obstetrics and Gynecology. 2006 Suppl: 40470. doi:10.1155/IDOG/2006/40470. PMC 1581465. PMID 16967912.
- ^ Kreimer AR, Clifford GM, Boyle P, Franceschi S (February 2005). "Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review". Cancer Epidemiology, Biomarkers & Prevention. 14 (2): 467–75. doi:10.1158/1055-9965.EPI-04-0551. PMID 15734974. S2CID 6643303.
- ^ Arbyn, Tommasino, Depuydt, Dillner (15 August 2014). "Are 20 human papillomavirus types causing cervical cancer?". The Journal of Pathology. 234 (4): 431–435. doi:10.1002/path.4424. PMID 25124771. S2CID 7775411.
- ^ Berrington de González A, Green J, et al. (International Collaboration of Epidemiological Studies of Cervical Cancer) (February 2007). "Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies". International Journal of Cancer. 120 (4): 885–91. doi:10.1002/ijc.22357. PMID 17131323. S2CID 33495556.
- ^ Denny LA, Franceschi S, de Sanjosé S, Heard I, Moscicki AB, Palefsky J (November 2012). "Human papillomavirus, human immunodeficiency virus and immunosuppression". Vaccine. 30 (Suppl 5): F168-74. doi:10.1016/j.vaccine.2012.06.045. PMID 23199960. Archived from the original on 8 November 2019. Retrieved 28 November 2019.
- ^ Dugué PA, Rebolj M, Garred P, Lynge E (January 2013). "Immunosuppression and risk of cervical cancer". Expert Review of Anticancer Therapy. 13 (1): 29–42. doi:10.1586/era.12.159. PMID 23259425. S2CID 26312718.
- ^ Willemsen, Anouk; Bravo, Ignacio G. (27 September 2018). "Origin and evolution of papillomavirus (onco)genes and genomes". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 374 (1773). bioRxiv 10.1101/428912. doi:10.1098/rstb.2018.0303. PMC 6501903. PMID 30955499.
- ^ Doorbar, John; Quint, Wim; Banks, Lawrence; Bravo, Ignacio G.; Stoler, Mark; Broker, Tom R.; Stanley, Margaret A. (November 2012). "The Biology and Life-Cycle of Human Papillomaviruses". Vaccine. 30: F55 – F70. doi:10.1016/j.vaccine.2012.06.083. ISSN 0264-410X. PMID 23199966.
- ^ Gagliardi, Alessia; Porter, Vanessa L.; Zong, Zusheng; Bowlby, Reanne; Titmuss, Emma; Namirembe, Constance; Griner, Nicholas B.; Petrello, Hilary; Bowen, Jay; Chan, Simon K.; Culibrk, Luka (August 2020). "Analysis of Ugandan cervical carcinomas identifies human papillomavirus clade–specific epigenome and transcriptome landscapes". Nature Genetics. 52 (8): 800–810. doi:10.1038/s41588-020-0673-7. ISSN 1061-4036. PMC 7498180. PMID 32747824.
- ^ Greenblatt RJ (2005). "Human papillomaviruses: Diseases, diagnosis, and a possible vaccine". Clinical Microbiology Newsletter. 27 (18): 139–145. doi:10.1016/j.clinmicnews.2005.09.001.
- ^ Abudula, Abulizi; Rouzi, Nuermanguli; Xu, Lixiu; Yang, Yun; Hasimu, Axiangu (2019). "Tissue-based metabolomics reveals potential biomarkers for cervical carcinoma and HPV infection". Bosnian Journal of Basic Medical Sciences. 20 (1): 78–87. doi:10.17305/bjbms.2019.4359. PMC 7029203. PMID 31465717.
- ^ Freitas LB, Chen Z, Muqui EF, Boldrini NA, Miranda AE, Spano LC, Burk RD (1 July 2014). "Human papillomavirus 16 non-European variants are preferentially associated with high-grade cervical lesions". PLOS ONE. 9 (7): e100746. Bibcode:2014PLoSO...9j0746F. doi:10.1371/journal.pone.0100746. PMC 4077691. PMID 24983739.
- ^ a b c d e Burd EM, Dean CL (August 2016). Hayden RT, Wolk DM, Carroll KC, Tang YC (eds.). "Human Papillomavirus". Microbiology Spectrum. Diagnostic Microbiology of the Immunocompromised Host. 4 (4) (Second ed.). American Society of Microbiology: 177–195. doi:10.1128/microbiolspec.dmih2-0001-2015. ISBN 978-1-55581-903-3. PMID 27726787.
- ^ Chin-Hong PV, Vittinghoff E, Cranston RD, Browne L, Buchbinder S, Colfax G, et al. (June 2005). "Age-related prevalence of anal cancer precursors in homosexual men: the EXPLORE study". Journal of the National Cancer Institute. 97 (12): 896–905. doi:10.1093/jnci/dji163. PMID 15956651.
- ^ Evans, David (10 June 2008). "AIDSmeds Web Exclusives: Pap Smears for Anal Cancer? — by David Evans". AIDSmeds.com. Archived from the original on 7 July 2011. Retrieved 29 August 2010.
- ^ Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Palefsky JM (June 2000). "Cost-effectiveness of screening for anal squamous intraepithelial lesions and anal cancer in human immunodeficiency virus-negative homosexual and bisexual men". The American Journal of Medicine. 108 (8): 634–41. doi:10.1016/S0002-9343(00)00349-1. PMID 10856411.
- ^ a b Elizabeth A. Van Dyne; S. Jane Henley; Mona Saraiya; Cheryll C. Thomas; Lauri E. Markowitz; Vicki B. Benard (24 August 2018). "Trends in Human Papillomavirus–Associated Cancers—United States, 1999–2015" (PDF). Morbidity and Mortality Weekly Report. Vol. 67, no. 33.
- ^ a b D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. (May 2007). "Case-control study of human papillomavirus and oropharyngeal cancer". The New England Journal of Medicine. 356 (19): 1944–56. doi:10.1056/NEJMoa065497. PMID 17494927. S2CID 18819678.
- ^ a b Ridge JA, Glisson BS, Lango MN, et al. "Head and Neck Tumors" Archived 20 July 2009 at the Wayback Machine in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer Management: A Multidisciplinary Approach Archived 4 October 2013 at the Wayback Machine. 11 ed. 2008.
- ^ Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. (May 2000). "Evidence for a causal association between human papillomavirus and a subset of head and neck cancers". Journal of the National Cancer Institute. 92 (9): 709–20. doi:10.1093/jnci/92.9.709. PMID 10793107.
- ^ Gillison ML (December 2006). "Human papillomavirus and prognosis of oropharyngeal squamous cell carcinoma: implications for clinical research in head and neck cancers". Journal of Clinical Oncology. 24 (36): 5623–5. doi:10.1200/JCO.2006.07.1829. PMID 17179099. S2CID 32491893.
- ^ Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, et al. (June 2015). "US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines". Journal of the National Cancer Institute. 107 (6): djv086. doi:10.1093/jnci/djv086. PMC 4838063. PMID 25925419.
- ^ Anantharaman D, Abedi-Ardekani B, Beachler DC, Gheit T, Olshan AF, Wisniewski K, et al. (May 2017). "Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer". International Journal of Cancer. 140 (9): 1968–1975. doi:10.1002/ijc.30608. hdl:2318/1634649. PMC 8969079. PMID 28108990. S2CID 34198821.
- ^ Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. (November 2011). "Human papillomavirus and rising oropharyngeal cancer incidence in the United States". Journal of Clinical Oncology. 29 (32): 4294–301. doi:10.1200/JCO.2011.36.4596. PMC 3221528. PMID 21969503.
- ^ Drake, Virginia; Fakhry, Carole; Windon, Melina J.; Stewart, C. Matthew; Akst, Lee; Hillel, Alexander; Chien, Wade; Ha, Patrick; Miles, Brett; Gourin, Christine G.; Mandal, Rajarsi; Mydlarz, Wojciech K.; Rooper, Lisa; Troy, Tanya; Yavvari, Siddhartha (11 January 2021). "Timing, number, and type of sexual partners associated with risk of oropharyngeal cancer". Cancer. 127 (7): 1029–1038. doi:10.1002/cncr.33346. ISSN 0008-543X. PMC 8035131. PMID 33426652.
- ^ Ernster JA, Sciotto CG, O'Brien MM, Finch JL, Robinson LJ, Willson T, Mathews M (December 2007). "Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus". The Laryngoscope. 117 (12): 2115–28. doi:10.1097/MLG.0b013e31813e5fbb. PMID 17891052. S2CID 38017888.
- ^ Lechner M, Frampton GM, Fenton T, Feber A, Palmer G, Jay A, et al. (2013). "Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV+ and HPV- tumors". Genome Medicine. 5 (5): 49. doi:10.1186/gm453. PMC 4064312. PMID 23718828.
- ^ "Lung Cancer Risk Rises in the Presence of HPV Antibodies". Archived from the original on 27 April 2012.
- ^ "Lung Cancer Patients More Likely to Have High-Risk Human Papillomavirus". NPIN. Archived from the original on 27 July 2012.
- ^ Syrjänen K, Syrjänen S, Kellokoski J, Kärjä J, Mäntyjärvi R (1989). "Human papillomavirus (HPV) type 6 and 16 DNA sequences in bronchial squamous cell carcinomas demonstrated by in situ DNA hybridization". Lung. 167 (1): 33–42. doi:10.1007/BF02714928. PMID 2537916. S2CID 2094038.
- ^ Carpagnano GE, Koutelou A, Natalicchio MI, Martinelli D, Ruggieri C, Di Taranto A, et al. (October 2011). "HPV in exhaled breath condensate of lung cancer patients". British Journal of Cancer. 105 (8): 1183–90. doi:10.1038/bjc.2011.354. PMC 3208494. PMID 21952627.
- ^ Klein F, Amin Kotb WF, Petersen I (July 2009). "Incidence of human papilloma virus in lung cancer". Lung Cancer. 65 (1): 13–8. doi:10.1016/j.lungcan.2008.10.003. PMID 19019488.
- ^ Moore M (12 November 2007). "Tree man 'who grew roots' may be cured". The Daily Telegraph. London. Archived from the original on 13 November 2007.
- ^ a b Patel T, Morrison LK, Rady P, Tyring S (2010). "Epidermodysplasia verruciformis and susceptibility to HPV". Disease Markers. 29 (3–4): 199–206. doi:10.1155/2010/345436. PMC 3835378. PMID 21178278.
- ^ "HPV and Cancer". National Cancer Institute. 15 May 2015. Archived from the original on 18 April 2017. Retrieved 18 April 2017.
- ^ a b Miyague AH, Mauad FM, de Paula Martins W, Benedetti AC, Ferreira AE, Mauad-Filho F (5 February 2019). "Ultrasound scan as a potential source of nosocomial and crossinfection: a literature review". Radiologia Brasileira. 48 (5): 319–23. doi:10.1590/0100-3984.2014.0002. PMC 4633077. PMID 26543284.
- ^ Hoyle, Alice; McGeeney, Ester (2019). Great Relationships and Sex Education. Taylor and Francis. ISBN 978-1-35118-825-8. Retrieved 11 July 2023.
- ^ a b Heymann MD (2015). Control of Communicable Diseases Manual (20th ed.). Washington D.C.: Apha Press. pp. 299–300. ISBN 978-0-87553-018-5.
- ^ a b Burchell AN, Winer RL, de Sanjosé S, Franco EL (August 2006). "Chapter 6: Epidemiology and transmission dynamics of genital HPV infection". Vaccine. 24 (Suppl 3): S3/52–61. doi:10.1016/j.vaccine.2006.05.031. PMID 16950018.
- ^ Schmitt M, Depuydt C, Benoy I, Bogers J, Antoine J, Arbyn M, Pawlita M (May 2013). "Prevalence and viral load of 51 genital human papillomavirus types and three subtypes". International Journal of Cancer. 132 (10): 2395–403. doi:10.1002/ijc.27891. PMID 23034864. S2CID 1316857.
- ^ Egendorf, Laura. Sexually Transmitted Diseases (At Issue Series). New York: Greenhaven Press, 2007.
- ^ a b Hernandez BY, Wilkens LR, Zhu X, Thompson P, McDuffie K, Shvetsov YB, et al. (June 2008). "Transmission of human papillomavirus in heterosexual couples". Emerging Infectious Diseases. 14 (6): 888–94. doi:10.3201/eid1406.070616. PMC 2600292. PMID 18507898.
- ^ a b Giuliano AR, Nielson CM, Flores R, Dunne EF, Abrahamsen M, Papenfuss MR, et al. (October 2007). "The optimal anatomic sites for sampling heterosexual men for human papillomavirus (HPV) detection: the HPV detection in men study". The Journal of Infectious Diseases. 196 (8): 1146–52. doi:10.1086/521629. PMC 3904649. PMID 17955432.
- ^ a b c d Winer RL, Hughes JP, Feng Q, Xi LF, Cherne S, O'Reilly S, et al. (July 2010). "Detection of genital HPV types in fingertip samples from newly sexually active female university students". Cancer Epidemiology, Biomarkers & Prevention. 19 (7): 1682–5. doi:10.1158/1055-9965.EPI-10-0226. PMC 2901391. PMID 20570905.
- ^ Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA (February 2003). "Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students". American Journal of Epidemiology. 157 (3): 218–26. doi:10.1093/aje/kwf180. PMID 12543621.
- ^ Tay SK (July 1995). "Genital oncogenic human papillomavirus infection: a short review on the mode of transmission" (Free full text). Annals of the Academy of Medicine, Singapore. 24 (4): 598–601. PMID 8849195. Archived from the original on 27 July 2012.
- ^ Pao CC, Tsai PL, Chang YL, Hsieh TT, Jin JY (March 1993). "Possible non-sexual transmission of genital human papillomavirus infections in young women". European Journal of Clinical Microbiology & Infectious Diseases. 12 (3): 221–2. doi:10.1007/BF01967118. PMID 8389707. S2CID 11548979.
- ^ Tay SK, Ho TH, Lim-Tan SK (August 1990). "Is genital human papillomavirus infection always sexually transmitted?" (Free full text). The Australian & New Zealand Journal of Obstetrics & Gynaecology. 30 (3): 240–2. doi:10.1111/j.1479-828X.1990.tb03223.x. PMID 2256864. S2CID 72353975. Archived from the original on 6 April 2016.
- ^ a b Sonnex C, Strauss S, Gray JJ (October 1999). "Detection of human papillomavirus DNA on the fingers of patients with genital warts". Sexually Transmitted Infections. 75 (5): 317–9. doi:10.1136/sti.75.5.317. PMC 1758241. PMID 10616355.
- ^ a b Hans Krueger; Gavin Stuart; Richard Gallagher; Dan Williams, Jon Kerner (12 April 2010). HPV and Other Infectious Agents in Cancer:Opportunities for Prevention and Public Health: Opportunities for Prevention and Public Health. Oxford University Press. p. 34. ISBN 978-0-19-973291-3. Archived from the original on 9 June 2013. Retrieved 24 December 2012.
- ^ Bodaghi S, Wood LV, Roby G, Ryder C, Steinberg SM, Zheng ZM (November 2005). "Could human papillomaviruses be spread through blood?". Journal of Clinical Microbiology. 43 (11): 5428–34. doi:10.1128/JCM.43.11.5428-5434.2005. PMC 1287818. PMID 16272465.
- ^ Chen AC, Keleher A, Kedda MA, Spurdle AB, McMillan NA, Antonsson A (October 2009). "Human papillomavirus DNA detected in peripheral blood samples from healthy Australian male blood donors" (PDF). Journal of Medical Virology. 81 (10): 1792–6. doi:10.1002/jmv.21592. hdl:10072/44445. PMID 19697401. S2CID 22918855.
- ^ "Eligibility Criteria by Topic - American Red Cross". Archived from the original on 1 January 2017.
- ^ a b c Watson RA (2005). "Human Papillomavirus: Confronting the Epidemic-A Urologist's Perspective". Reviews in Urology. 7 (3): 135–44. PMC 1477576. PMID 16985824.
- ^ Guan J, Bywaters SM, Brendle SA, Ashley RE, Makhov AM, Conway JF, et al. (February 2017). "Cryoelectron Microscopy Maps of Human Papillomavirus 16 Reveal L2 Densities and Heparin Binding Site". Structure. 25 (2): 253–263. doi:10.1016/j.str.2016.12.001. PMID 28065506.
- ^ a b c Schiller JT, Day PM, Kines RC (June 2010). "Current understanding of the mechanism of HPV infection". Gynecologic Oncology. 118 (1 Suppl): S12–7. doi:10.1016/j.ygyno.2010.04.004. PMC 3493113. PMID 20494219.
- ^ a b Scheurer ME, Tortolero-Luna G, Adler-Storthz K (2005). "Human papillomavirus infection: biology, epidemiology, and prevention". International Journal of Gynecological Cancer. 15 (5): 727–46. doi:10.1111/j.1525-1438.2005.00246.x. PMID 16174218. S2CID 23849159.
- ^ Woodham AW, Da Silva DM, Skeate JG, Raff AB, Ambroso MR, Brand HE, et al. (2012). "The S100A10 subunit of the annexin A2 heterotetramer facilitates L2-mediated human papillomavirus infection". PLOS ONE. 7 (8): e43519. Bibcode:2012PLoSO...743519W. doi:10.1371/journal.pone.0043519. PMC 3425544. PMID 22927980.
- ^ Raff AB, Woodham AW, Raff LM, Skeate JG, Yan L, Da Silva DM, et al. (June 2013). "The evolving field of human papillomavirus receptor research: a review of binding and entry". Journal of Virology. 87 (11): 6062–72. doi:10.1128/JVI.00330-13. PMC 3648114. PMID 23536685.
- ^ Chen Z, Schiffman M, Herrero R, Desalle R, Anastos K, Segondy M, et al. (2011). "Evolution and taxonomic classification of human papillomavirus 16 (HPV16)-related variant genomes: HPV31, HPV33, HPV35, HPV52, HPV58 and HPV67". PLOS ONE. 6 (5): e20183. Bibcode:2011PLoSO...620183C. doi:10.1371/journal.pone.0020183. PMC 3103539. PMID 21673791.
- ^ Zuna RE, Tuller E, Wentzensen N, Mathews C, Allen RA, Shanesmith R, et al. (October 2011). "HPV16 variant lineage, clinical stage, and survival in women with invasive cervical cancer". Infectious Agents and Cancer. 6: 19. doi:10.1186/1750-9378-6-19. PMC 3226431. PMID 22035468.
- ^ a b Mirabello, Lisa; Clarke, Megan; Nelson, Chase; Dean, Michael; Wentzensen, Nicolas; Yeager, Meredith; Cullen, Michael; Boland, Joseph; NCI HPV Workshop; Schiffman, Mark; Burk, Robert (13 February 2018). "The Intersection of HPV Epidemiology, Genomics and Mechanistic Studies of HPV-Mediated Carcinogenesis". Viruses. 10 (2): 80. doi:10.3390/v10020080. ISSN 1999-4915. PMC 5850387. PMID 29438321.
- ^ a b c d e Nikolaidis, Marios; Tsakogiannis, Dimitris; Bletsa, Garyfalia; Mossialos, Dimitris; Kottaridi, Christine; Iliopoulos, Ioannis; Markoulatos, Panayotis; Amoutzias, Grigoris D. (14 October 2021). "HPV16-Genotyper: A Computational Tool for Risk-Assessment, Lineage Genotyping and Recombination Detection in HPV16 Sequences, Based on a Large-Scale Evolutionary Analysis". Diversity. 13 (10): 497. Bibcode:2021Diver..13..497N. doi:10.3390/d13100497. ISSN 1424-2818.
- ^ Mirabello, Lisa; Yeager, Meredith; Cullen, Michael; Boland, Joseph F.; Chen, Zigui; Wentzensen, Nicolas; Zhang, Xijun; Yu, Kai; Yang, Qi; Mitchell, Jason; Roberson, David (September 2016). "HPV16 Sublineage Associations With Histology-Specific Cancer Risk Using HPV Whole-Genome Sequences in 3200 Women". Journal of the National Cancer Institute. 108 (9): djw100. doi:10.1093/jnci/djw100. ISSN 0027-8874. PMC 5939630. PMID 27130930.
- ^ Jiang, Mingjun; Xi, Long Fu; Edelstein, Zoe R.; Galloway, Denise A.; Olsem, Gary J.; Lin, William Chun-Che; Kiviat, Nancy B. (November 2009). "Identification of recombinant human papillomavirus type 16 variants". Virology. 394 (1): 8–11. doi:10.1016/j.virol.2009.08.040. PMC 2769496. PMID 19758676.
- ^ Zanier K, Charbonnier S, Sidi AO, McEwen AG, Ferrario MG, Poussin-Courmontagne P, et al. (February 2013). "Structural basis for hijacking of cellular LxxLL motifs by papillomavirus E6 oncoproteins". Science. 339 (6120): 694–8. Bibcode:2013Sci...339..694Z. doi:10.1126/science.1229934. PMC 3899395. PMID 23393263.
- ^ Ganguly N, Parihar SP (March 2009). "Human papillomavirus E6 and E7 oncoproteins as risk factors for tumorigenesis". Journal of Biosciences. 34 (1): 113–23. doi:10.1007/s12038-009-0013-7. PMID 19430123. S2CID 8770549.
- ^ Zheng ZM, Baker CC (September 2006). "Papillomavirus genome structure, expression, and post-transcriptional regulation". Frontiers in Bioscience. 11: 2286–302. doi:10.2741/1971. PMC 1472295. PMID 16720315.
- ^ Tang S, Tao M, McCoy JP, Zheng ZM (May 2006). "The E7 oncoprotein is translated from spliced E6*I transcripts in high-risk human papillomavirus type 16- or type 18-positive cervical cancer cell lines via translation reinitiation". Journal of Virology. 80 (9): 4249–63. doi:10.1128/JVI.80.9.4249-4263.2006. PMC 1472016. PMID 16611884.
- ^ Alison A. McBride (18 March 2017). "Mechanisms and strategies of papillomavirus replication". Biological Chemistry. 398 (8): 919–927. doi:10.1515/HSZ-2017-0113. ISSN 1431-6730. PMID 28315855. Wikidata Q39186071.
- ^ a b Chaturvedi A, Gillison ML (4 March 2010). "Human Papillomavirus and Head and Neck Cancer". In Andrew F. Olshan (ed.). Epidemiology, Pathogenesis, and Prevention of Head and Neck Cancer (1st ed.). New York: Springer. pp. 87–116. doi:10.1007/978-1-4419-1472-9_5. ISBN 978-1-4419-1471-2.
- ^ Münger K, Howley PM (November 2002). "Human papillomavirus immortalization and transformation functions". Virus Research. 89 (2): 213–28. doi:10.1016/S0168-1702(02)00190-9. PMID 12445661.
- ^ Conway MJ, Alam S, Ryndock EJ, Cruz L, Christensen ND, Roden RB, Meyers C (October 2009). "Tissue-spanning redox gradient-dependent assembly of native human papillomavirus type 16 virions". Journal of Virology. 83 (20): 10515–26. doi:10.1128/JVI.00731-09. PMC 2753102. PMID 19656879.
- ^ Bryan JT, Brown DR (March 2001). "Transmission of human papillomavirus type 11 infection by desquamated cornified cells". Virology. 281 (1): 35–42. doi:10.1006/viro.2000.0777. PMID 11222093.
- ^ Rampias T, Boutati E, Pectasides E, Sasaki C, Kountourakis P, Weinberger P, Psyrri A (March 2010). "Activation of Wnt signaling pathway by human papillomavirus E6 and E7 oncogenes in HPV16-positive oropharyngeal squamous carcinoma cells". Molecular Cancer Research. 8 (3): 433–43. doi:10.1158/1541-7786.MCR-09-0345. PMID 20215420. S2CID 19411033.
- ^ Giuliano AR, Lu B, Nielson CM, Flores R, Papenfuss MR, Lee JH, et al. (September 2008). "Age-specific prevalence, incidence, and duration of human papillomavirus infections in a cohort of 290 US men". The Journal of Infectious Diseases. 198 (6): 827–35. doi:10.1086/591095. PMID 18657037.
- ^ EHPV, archived from the original on 17 December 2014
- ^ Schiffman M, Castle PE (August 2003). "Human papillomavirus: epidemiology and public health" [1 January 2017]. Archives of Pathology & Laboratory Medicine. 127 (8): 930–4. doi:10.5858/2003-127-930-HPEAPH. PMID 12873163. Archived from the original on 14 April 2013.
- ^ "HPV | Human Papillomavirus | Pap Smear | MedlinePlus". Retrieved 7 November 2018.
- ^ Saslow, D; Solomon, D; Lawson, HW; Killackey, M; Kulasingam, SL; Cain, J; Garcia, FA; Moriarty, AT; Waxman, AG; Wilbur, DC; Wentzensen, N; Downs LS, Jr; Spitzer, M; Moscicki, AB; Franco, EL; Stoler, MH; Schiffman, M; Castle, PE; Myers, ER; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee (May 2012). "American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer". CA: A Cancer Journal for Clinicians. 62 (3): 147–72. doi:10.3322/caac.21139. PMC 3801360. PMID 22422631.
- ^ Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, et al. (March 2017). "Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening". CA: A Cancer Journal for Clinicians. 67 (2): 100–121. doi:10.3322/caac.21392. PMID 28170086. S2CID 37359995.
- ^ "National Cancer Institute Fact Sheet: HPV and Cancer". Cancer.gov. Archived from the original on 31 October 2013. Retrieved 23 October 2013.
- ^ Pan C, Issaeva N, Yarbrough WG (2018). "HPV-driven oropharyngeal cancer: current knowledge of molecular biology and mechanisms of carcinogenesis". Cancers of the Head & Neck. 3: 12. doi:10.1186/s41199-018-0039-3. PMC 6460765. PMID 31093365.
- ^ a b c d e Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR (October 2006). "Prevalence of HPV infection among men: A systematic review of the literature". The Journal of Infectious Diseases. 194 (8): 1044–57. doi:10.1086/507432. PMID 16991079.
- ^ Burchell AN, Richardson H, Mahmud SM, Trottier H, Tellier PP, Hanley J, et al. (March 2006). "Modeling the sexual transmissibility of human papillomavirus infection using stochastic computer simulation and empirical data from a cohort study of young women in Montreal, Canada". American Journal of Epidemiology. 163 (6): 534–43. doi:10.1093/aje/kwj077. PMID 16421235.
- ^ Kim JJ (October 2007). "Vaccine policy analyses can benefit from natural history studies of human papillomavirus in men". The Journal of Infectious Diseases. 196 (8): 1117–9. doi:10.1086/521199. PMID 17955427.
- ^ "FAQs for Men". thehpvtest.com. Archived from the original on 5 September 2012. Retrieved 24 August 2012.
- ^ a b Nicolau SM, Camargo CG, Stávale JN, Castelo A, Dôres GB, Lörincz A, de Lima GR (February 2005). "Human papillomavirus DNA detection in male sexual partners of women with genital human papillomavirus infection". Urology. 65 (2): 251–5. doi:10.1016/j.urology.2004.09.031. PMID 15708032.
- ^ a b c Aguilar LV, Lazcano-Ponce E, Vaccarella S, Cruz A, Hernández P, Smith JS, et al. (February 2006). "Human papillomavirus in men: comparison of different genital sites". Sexually Transmitted Infections. 82 (1): 31–3. doi:10.1136/sti.2005.015131. PMC 2563819. PMID 16461598.
- ^ a b c Weaver BA, Feng Q, Holmes KK, Kiviat N, Lee SK, Meyer C, et al. (February 2004). "Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men". The Journal of Infectious Diseases. 189 (4): 677–85. doi:10.1086/381395. PMID 14767822.
- ^ Hernandez BY, McDuffie K, Goodman MT, Wilkens LR, Thompson P, Zhu X, et al. (February 2006). "Comparison of physician- and self-collected genital specimens for detection of human papillomavirus in men". Journal of Clinical Microbiology. 44 (2): 513–7. doi:10.1128/JCM.44.2.513-517.2006. PMC 1392697. PMID 16455906.
- ^ Ogilvie GS, Taylor DL, Achen M, Cook D, Krajden M (June 2009). "Self-collection of genital human papillomavirus specimens in heterosexual men". Sexually Transmitted Infections. 85 (3): 221–5. doi:10.1136/sti.2008.033068. PMID 19066196. S2CID 24410167.
- ^ Petignat P, Faltin DL, Bruchim I, Tramèr MR, Franco EL, Coutlée F (May 2007). "Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis". Gynecologic Oncology. 105 (2): 530–5. doi:10.1016/j.ygyno.2007.01.023. PMID 17335880.
- ^ Partridge JM, Hughes JP, Feng Q, Winer RL, Weaver BA, Xi LF, et al. (October 2007). "Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students". The Journal of Infectious Diseases. 196 (8): 1128–36. doi:10.1086/521192. PMID 17955430.
- ^ "HPV and Men — CDC Fact Sheet". Centers for Disease Control and Prevention. Centers for Disease Control and Prevention (CDC). 3 April 2008. Archived from the original on 17 October 2009. Retrieved 13 November 2009.
- ^ "Human Papillomavirus (HPV) and Men: Questions and Answers". 2007. Archived from the original on 14 September 2008. Retrieved 10 September 2008.
Currently, in Canada there is an HPV DNA test approved for women but not for men.
- ^ "What Men Need to Know About HPV". 2006. Archived from the original on 7 April 2007. Retrieved 4 April 2007.
There is currently no FDA-approved test to detect HPV in men. That is because an effective, reliable way to collect a sample of male genital skin cells, which would allow detection of HPV, has yet to be developed.
- ^ Storey R, Joh J, Kwon A, Jenson AB, Ghim SJ, Kloecker GH (2013). "Detection of Immunoglobulin G against E7 of Human Papillomavirus in Non-Small-Cell Lung Cancer". Journal of Oncology. 2013: 240164. doi:10.1155/2013/240164. PMC 3603668. PMID 23533408.
- ^ Rocha-Zavaleta L, Ambrosio JP, de Lourdes Mora-Garcia M, Cruz-Talonia F, Hernandez-Montes J, Weiss-Steider B, et al. (September 2004). "Detection of antibodies against a human papillomavirus (HPV) type 16 peptide that differentiate high-risk from low-risk HPV-associated low-grade squamous intraepithelial lesions". The Journal of General Virology. 85 (Pt 9): 2643–50. doi:10.1099/vir.0.80077-0. PMID 15302958.
- ^ Bolhassani A, Zahedifard F, Taslimi Y, Taghikhani M, Nahavandian B, Rafati S (November 2009). "Antibody detection against HPV16 E7 & GP96 fragments as biomarkers in cervical cancer patients" (PDF). The Indian Journal of Medical Research. 130 (5): 533–41. PMID 20090101. Archived from the original (PDF) on 16 December 2010. Retrieved 18 March 2014.
- ^ Fitzgerald K (18 June 2013). "Blood Test May Detect Sexually Transmitted Throat Cancer". Medical News Today. Archived from the original on 7 April 2014. Retrieved 18 March 2014.
- ^ "HPV (Human Papilloma Virus) Diagnosis and Tests". Cleveland Clinic. 18 September 2018. Archived from the original on 1 August 2020. Retrieved 8 February 2019.
- ^ "FDA approves Gardasil 9 for prevention of certain cancers caused by five additional types of HPV". Food and Drug Administration. 10 December 2014. Archived from the original on 10 January 2015. Retrieved 8 March 2015.
- ^ "Human Papillomavirus Epidemiology and Prevention of Vaccine-Preventable Diseases". CDC. Archived from the original on 3 February 2014. Retrieved 30 January 2014.
- ^ "Human papillomavirus vaccines. WHO position paper" (PDF). Relevé Épidémiologique Hebdomadaire. 84 (15): 118–31. April 2009. PMID 19360985. Archived (PDF) from the original on 24 December 2010.
- ^ a b c Koliopoulos G, Nyaga VN, Santesso N, Bryant A, Martin-Hirsch PP, Mustafa RA, et al. (August 2017). "Cytology versus HPV testing for cervical cancer screening in the general population". The Cochrane Database of Systematic Reviews. 8 (7): CD008587. doi:10.1002/14651858.CD008587.pub2. PMC 6483676. PMID 28796882.
- ^ "CDC recommends only two HPV shots for younger adolescents". Centers for Disease Control and Prevention. 20 October 2016. Archived from the original on 23 March 2017. Retrieved 24 March 2017.
- ^ a b Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER (March 2007). "Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP)". MMWR. Recommendations and Reports. 56 (RR-2): 1–24. PMID 17380109. Archived from the original on 20 May 2017.
- ^ Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, et al. (May 2013). "Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial". JAMA. 309 (17): 1793–802. doi:10.1001/jama.2013.1625. PMID 23632723.
- ^ "HPV Vaccine Information For Young Women". Centers for Disease Control and Prevention. 3 January 2017. Archived from the original on 25 March 2017. Retrieved 24 March 2017.
- ^ "HPV Virus: Information About Human Papillomavirus". WebMD. Archived from the original on 8 March 2008.
- ^ "Gardasil patient information leaflet" (PDF). April 2015. Retrieved 11 July 2018.
- ^ Deleré Y, Wichmann O, Klug SJ, van der Sande M, Terhardt M, Zepp F, Harder T (September 2014). "The efficacy and duration of vaccine protection against human papillomavirus: a systematic review and meta-analysis". Deutsches Ärzteblatt International. 111 (35–36): 584–91. doi:10.3238/arztebl.2014.0584. PMC 4174682. PMID 25249360.
- ^ "FDA approves Gardasil 9 for prevention of certain cancers caused by five additional types of HPV". Food and Drug Administration (press release). 10 December 2014. Archived from the original on 10 January 2015. Retrieved 28 February 2015.
- ^ "CDC — Condom Effectiveness — Male Latex Condoms and Sexually Transmitted Diseases". Centers for Disease Control and Prevention (CDC). 22 October 2009. Archived from the original on 17 October 2009. Retrieved 23 October 2009.
- ^ a b Meyers J, Ryndock E, Conway MJ, Meyers C, Robison R (June 2014). "Susceptibility of high-risk human papillomavirus type 16 to clinical disinfectants". The Journal of Antimicrobial Chemotherapy. 69 (6): 1546–50. doi:10.1093/jac/dku006. PMC 4019329. PMID 24500190.
- ^ Liu Z, Rashid T, Nyitray AG (February 2016). "Penises not required: a systematic review of the potential for human papillomavirus horizontal transmission that is non-sexual or does not include penile penetration". Sexual Health. 13 (1): 10–21. doi:10.1071/sh15089. PMID 26433493. S2CID 20937073.
- ^ Sabeena S, Bhat P, Kamath V, Arunkumar G (March 2017). "Possible non-sexual modes of transmission of human papilloma virus". The Journal of Obstetrics and Gynaecology Research. 43 (3): 429–435. doi:10.1111/jog.13248. PMID 28165175. S2CID 39387099.
- ^ a b c HSE Quality Improvement Division — Decontamination Safety Programme (January 2017). Health Service Executive Guidance for Decontamination of Semi-critical Ultrasound Probes; Semi-invasive and Non-invasive Ultrasound Probes (PDF) (Report). Department of Health United Kingdom. QPSD-GL-028-1. Archived from the original (PDF) on 1 August 2020. Retrieved 8 February 2019.
- ^ "Recommendations for Cleaning and Disinfection in Medical Ultrasound to Prevent Human Papillomavirus (HPV) Transmission" (PDF). Provincial Infection Control Network of British Columbia. 2 June 2016.
- ^ "Reprocessing Requirements for Ultrasound Probes" (PDF). College of Physicians and Surgeons of British Columbia. December 2017.
- ^ a b "Genital HPV Infection Fact Sheet". Centers for Disease Control and Prevention (CDC). 10 April 2008. Archived from the original on 11 September 2012. Retrieved 13 November 2009.
- ^ "HPV Vaccine Information For Young Women". Centers for Disease Control and Prevention (CDC). 26 June 2008. Archived from the original on 26 October 2009. Retrieved 13 November 2009.
- ^ American Cancer Society. "What Are the Risk Factors for Cervical Cancer?". Archived from the original on 19 February 2008. Retrieved 21 February 2008.
- ^ "Cure for HPV". Webmd.com. Archived from the original on 18 August 2010. Retrieved 29 August 2010.
- ^ Gilbert LK, Alexander L, Grosshans JF, Jolley L (March 2003). "Answering frequently asked questions about HPV". Sexually Transmitted Diseases. 30 (3): 193–4. doi:10.1097/00007435-200303000-00002. PMID 12616133.
- ^ "Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis". Centers for Disease Control and Prevention. Archived from the original on 16 November 2015. Retrieved 23 October 2015.
- ^ Desai M, Woodhall SC, Nardone A, Burns F, Mercey D, Gilson R (August 2015). "Active recall to increase HIV and STI testing: a systematic review". Sexually Transmitted Infections. 91 (5): 314–23. doi:10.1136/sextrans-2014-051930. PMID 25759476. S2CID 663971.
- ^


 French
French Deutsch
Deutsch