Isatin
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 1H-Indole-2,3-dione | |
| Identifiers | |
3D model (JSmol) | |
| 383659 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.889 |
| EC Number |
|
| 165206 | |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C8H5NO2 | |
| Molar mass | 147.1308 g/mol |
| Appearance | Orange-red solid |
| Melting point | 200 °C (392 °F; 473 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Isatin, also known as tribulin, is an organic compound derived from indole with formula C8H5NO2. The compound was first obtained by Otto Linné Erdman[1] and Auguste Laurent[2] in 1840 as a product from the oxidation of indigo dye by nitric acid and chromic acids.
Isatin is a well-known natural product which can be found in plants of the genus Isatis, in Couroupita guianensis,[3][4] and also in humans, as a metabolic derivative of adrenaline.[5]
It looks like a red-orange powder, and it is usually employed as building block for the synthesis of a wide variety of biologically active compounds including antitumorals,[6] antivirals,[7] anti-HIVs,[8] and antituberculars.[9]
The isatin core is also responsible for the color of “Maya blue” and “Maya yellow” dyes.[10]
Synthesis
[edit]Sandmeyer methodology
[edit]The Sandmeyer methodology is the oldest and straightforward way for the synthesis of isatin.[11] The method involves the condensation between chloral hydrate and a primary arylamine (e.g. aniline), in the presence of hydroxylamine hydrochloride, in aqueous sodium sulfate to form an α‐isonitrosoacetanilide. Isolation of this intermediate and subsequent electrophilic cyclization promoted by strong acids (e.g. sulfuric acid) furnishes isatin in >75% yield.

Stolle methodology
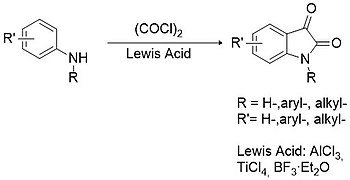
[edit]The Stolle procedure is considered the best alternative to Sandmeyer methodology for the synthesis of both substituted and unsubstituted isatins.[12] In this case primary or secondary arylamines are condensed with oxalyl chloride to form a chlorooxalylanilide intermediate which can then cyclize in the presence of a Lewis acid (e.g. aluminium trichloride, titanium tetrachloride, boron trifluoride, etc.).
Other procedures
[edit]More recent approaches to the synthesis of N-substituted isatins involves the direct oxidation of commercially available, substituted indoles or oxindoles with different oxidizing agents such as TBHP,[13] IBX-SO3K,[14] tBuONO[15] etc.

Reactivity
[edit]The presence of an aromatic ring, a ketone and a γ-lactam moiety, gives to isatin the rare potential to be used as both an electrophile and a nucleophile: indeed, it undergoes an enormous number of reactions, such as N-substitutions, electrophilic aromatic substitution at positions C-5 and C-7 of the phenyl ring, nucleophilic additions onto the C-3 carbonyl group, chemoselective reductions, oxidations, ring-expansions and spiro-annulations. Because of this unique reactivity, isatin is considered one of the most valuable building blocks in organic synthesis.
N-Substitution
[edit]The N-functionalization of the isatin core can be readily obtained by the deprotonation of the amino moiety, forming the corresponding sodium or potassium salt, and subsequent addition of an electrophile (e.g. alkyl or acyl halides).

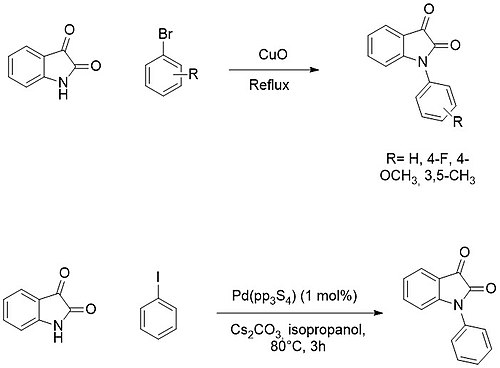
On the other hand, N-arylation is usually achieved by cross-coupling reactions with aryl halides using copper or palladium catalysts.[16][17]
Ring expansion
[edit]In the field of organic synthesis, ring expansions are considered valuable reactions since they allow the obtainment medium-size ring (7-9 atoms) which are difficult to synthesize through "classical" methods.[18]
To date, only few articles concerning the ring expansion of isatin derivatives has been reported. The first one is an acid-catalyzed one-pot multicomponent reaction involving isatins, aminouracils, and isooxazolones to form isoxazoquinolines, important scaffolds in medicinal chemistry.[19]

In another one-pot multicomponent reaction, a unique two-carbon expansion has been achieved by reacting isatin with indene-1,3-dione and N-substituted pyridinium bromide to form dibenzo[b,d]azepin-6-ones.[20]

C-2/C-3 nucleophilic addition
[edit]Isatin suffers nucleophilic addition on carbonyls at C-2 and C-3 positions. The regioselectivity of the process strongly depends both on the substrate (properties of the substituents on the isatin core, especially those bonded to the nitrogen atom) and the reaction conditions (solvent, temperature etc.). In some cases the nucleophilic addition could be followed by secondary reactions (e.g. cyclization, ring expansion, ring opening etc.)

Oxidation
[edit]The oxidation of isatin using hydrogen peroxide (Baeyer–Villiger oxidation) or chromic anhydride yields isatoic anhydride,[22][23][24] a compound widely used either in herbicide products and in medicinal chemistry. The use of peroxydisulfuric acid gives rise to 1,4‑benzoxazine compounds.[22]

Dimerization
[edit]Dimerization of isatin with KBH4 in methanol yield Indirubin.[25] This represent the indigo pigment's red component and a highly effective cytotoxic compound.

Reduction
[edit]Reduction of the non-amide carbonyl group obviously occurs to give oxindole, respectively.
See also
[edit]References
[edit]- ^ Erdmann, Otto Linné (1840). "Untersuchungen über den Indigo". Journal für Praktische Chemie. 19 (1): 321–362. doi:10.1002/prac.18400190161.
- ^ Laurent, Auguste (1840). "Recherches sur l'indigo". Annales de Chimie et de Physique. 3 (3): 393–434.
- ^ Pinto, A. C. (2001). "The chemistry of isatins: a review from 1975 to 1999". J. Braz. Chem. Soc. 12 (3): 273. doi:10.1590/S0103-50532001000300002.
- ^ Bergman, J. (1988). "The structure and properties of some indolic constituents in Couroupita guianensis aubl". Tetrahedron. 41 (14): 2879. doi:10.1016/S0040-4020(01)96609-8.
- ^ Chiyanzu, I. (2003). "Synthesis and evaluation of isatins and thiosemicarbazone derivatives against cruzain, falcipain-2 and rhodesain". Bioorg. Med. Chem. Lett. 13 (20): 3527–30. doi:10.1016/S0960-894X(03)00756-X. PMID 14505663.
- ^ Mallamo, J.P. (2006). "Structure-guided identification of novel VEGFR-2 kinase inhibitors via solution phase parallel synthesis". Bioorg. Med. Chem. Lett. 16 (8): 2158–62. doi:10.1016/j.bmcl.2006.01.063. PMID 16460933.
- ^ He, Y. (2006). "Design, synthesis, and biological evaluations of novel oxindoles as HIV-1 non-nucleoside reverse transcriptase inhibitors". Bioorg. Med. Chem. Lett. 16 (8): 2109–12. doi:10.1016/j.bmcl.2006.01.066. PMID 16464578.
- ^ Sriram, D. (2005). "Synthesis and evaluation of anti-HIV activity of isatin beta-thiosemicarbazone derivatives". Bioorg. Med. Chem. Lett. 15 (20): 4451–5. doi:10.1016/j.bmcl.2005.07.046. PMID 16115762.
- ^ Bin-Jubair, F.A.S. (2010). "Anti-Tubercular activity of Isatin and Derivatives". Int. J. Res. Pharm. Sci. 1: 113.
- ^ Vuzquez de Agredos-Pascual, M.L. (2011). "From Maya Blue to "Maya Yellow": A Connection between Ancient Nanostructured Materials from the Voltammetry of Microparticles". Angew. Chem. Int. Ed. 50 (25): 5741–4. doi:10.1002/anie.201100921. PMID 21557419.
- ^ Sandmeyer, T. (1919). "Über Isonitrosoacetanilide und deren Kondensation zu Isatinen". Helv. Chim. Acta. 2: 234. doi:10.1002/hlca.19190020125.
- ^ Stollé, R. (1922). "Über N-substituierte Oxindole und Isatine". J. Prakt. Chem. (In German). 105 (1): 137–148. doi:10.1002/prac.19221050111.
- ^ Ji, S.J. (2014). "I2/TBHP-Catalyzed Chemoselective Amination of Indoles". Org. Lett. 16: 3094–3097.
- ^ Kirsch, S.F. (2015). "Synthesis of Isatins through Direct Oxidation of Indoles with IBX-SO3K/NaI". Synthesis. 47 (13): 1937–1943. doi:10.1055/s-0034-1380517.
- ^ Wei, W.T. (2018). "Synthesis of Indoline-2,3-diones by Radical Coupling of Indolin-2-ones with tert-Butyl Hydroperoxide". Synlett. 29 (2): 215–218. doi:10.1055/s-0036-1589106.
- ^ Coppola, Gary M. (September 1987). "Arylation of isatins. A direct route to -arylisatoic anhydrides". Journal of Heterocyclic Chemistry. 24 (5): 1249–1251. doi:10.1002/jhet.5570240503.
- ^ Majumder, Arpi; Gupta, Ragini; Mandal, Mrinmay; Babu, Madhu; Chakraborty, Debashis (April 2015). "Air-stable palladium(0) phosphine sulfide catalysts for Ullmann-type C–N and C–O coupling reactions". Journal of Organometallic Chemistry. 781: 23–34. doi:10.1016/j.jorganchem.2014.11.018.
- ^ Donald, James R.; Unsworth, William P. (3 July 2017). "Ring-Expansion Reactions in the Synthesis of Macrocycles and Medium-Sized Rings". Chemistry - A European Journal. 23 (37): 8780–8799. doi:10.1002/chem.201700467. PMID 28295709.
- ^ Poomathi, Nataraj; Mayakrishnan, Sivakalai; Muralidharan, Doraiswamy; Srinivasan, Rajagopal; Perumal, Paramasivan T. (2015). "Reaction of isatins with 6-amino uracils and isoxazoles: isatin ring-opening vs. annulations and regioselective synthesis of isoxazole fused quinoline scaffolds in water". Green Chemistry. 17 (6): 3362–3372. doi:10.1039/c5gc00006h.
- ^ Shi, Rong-Guo; Wang, Xiao-Hua; Liu, Ruzhang; Yan, Chao-Guo (2016). "Two-carbon ring expansion of isatin: a convenient construction of a dibenzo[b,d]azepinone scaffold". Chemical Communications. 52 (37): 6280–6283. doi:10.1039/c6cc00525j. PMID 27079548. S2CID 36547699.
- ^ Bergman, Jan; Stålhandske, Claes; Vallberg, Hans (1997). "Studies of the Reaction between Indole-2,3-diones (Isatins) and Secondary Aliphatic Amines" (PDF). Acta Chemica Scandinavica. 51: 753–759. doi:10.3891/acta.chem.scand.51-0753.
- ^ a b Reissenweber, Gernot; Mangold, Dietrich (March 1980). "Oxidation of Isatins to Isatoic Anhydrides and 2,3-Dioxo-1,4-benzoxazines". Angewandte Chemie International Edition in English. 19 (3): 222–223. doi:10.1002/anie.198002221.
- ^ Yang, Shuangshuang; Li, Xishuai; Hu, Fangfang; Li, Yinlong; Yang, Yunyun; Yan, Junkai; Kuang, Chunxiang; Yang, Qing (25 October 2013). "Discovery of Tryptanthrin Derivatives as Potent Inhibitors of Indoleamine 2,3-Dioxygenase with Therapeutic Activity in Lewis Lung Cancer (LLC) Tumor-Bearing Mice". Journal of Medicinal Chemistry. 56 (21): 8321–8331. doi:10.1021/jm401195n. PMID 24099220.
- ^ Bao, Yajie; Yan, Yizhe; Xu, Kun; Su, Jihu; Zha, Zhenggen; Wang, Zhiyong (20 April 2015). "Copper-Catalyzed Radical Methylation/C–H Amination/Oxidation Cascade for the Synthesis of Quinazolinones". The Journal of Organic Chemistry. 80 (9): 4736–4742. doi:10.1021/acs.joc.5b00191. PMID 25849218.
- ^ Wang, Cuiling; Yan, Jiaxu; Du, Mo; Burlison, Joseph A.; Li, Chi; Sun, Yanni; Zhao, Danqing; Liu, Jianli (May 2017). "One step synthesis of indirubins by reductive coupling of isatins with KBH 4". Tetrahedron. 73 (19): 2780–2785. doi:10.1016/j.tet.2017.03.077.
Reviews
[edit]- Popp, Prank D. (1975). "The Chemistry of Isatin". Advances in Heterocyclic Chemistry Volume 18. Vol. 18. pp. 1–58. doi:10.1016/S0065-2725(08)60127-0. ISBN 9780120206186.
- Silva, Joaquim F. M. da; Garden, Simon J.; Pinto, Angelo C. (June 2001). "The chemistry of isatins: a review from 1975 to 1999". Journal of the Brazilian Chemical Society. 12 (3): 273–324. doi:10.1590/S0103-50532001000300002.
- Mesropyan, E. G.; Avetisyan, A. A. (2009). "New isatin derivatives". Russian Journal of Organic Chemistry. 45 (11): 1583. doi:10.1134/S1070428009110013. S2CID 97341279.
- Varun, Varun; Sonam, Sonam; Kakkar, Rita (2019). "Isatin and its derivatives: a survey of recent syntheses, reactions, and applications". MedChemComm. 10 (3): 351–368. doi:10.1039/C8MD00585K. PMC 6438150. PMID 30996856.
External links
[edit]- . Encyclopædia Britannica. Vol. 14 (11th ed.). 1911. pp. 865–866.


 French
French Deutsch
Deutsch