JWH-359
 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H36O2 |
| Molar mass | 356.550 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
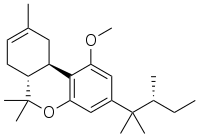
JWH-359 is a dibenzopyran "classical" cannabinoid drug, which is a potent and selective CB2 receptor agonist, with a Ki of 13.0 nM and selectivity of around 220 times for CB2 over CB1 receptors. It is related to other dibenzopyran CB2 agonists such as JWH-133 and L-759,656 but with a chiral side chain which has made it useful for mapping the shape of the CB2 binding site.[1][2] It was discovered by, and named after, John W. Huffman.
References
[edit]- ^ Huffman JW, Bushell SM, Joshi SN, Wiley JL, Martin BR (January 2006). "Enantioselective synthesis of 1-methoxy- and 1-deoxy-2'-methyl-delta8-tetrahydrocannabinols: new selective ligands for the CB2 receptor". Bioorganic & Medicinal Chemistry. 14 (1): 247–62. doi:10.1016/j.bmc.2005.08.013. PMID 16165365.
- ^ Marriott KS, Huffman JW (2008). "Recent advances in the development of selective ligands for the cannabinoid CB(2) receptor". Current Topics in Medicinal Chemistry. 8 (3): 187–204. doi:10.2174/156802608783498014. PMID 18289088.


 French
French Deutsch
Deutsch