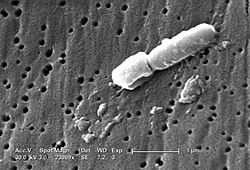
Klebsiella
Klebsiella is a genus of Gram-negative, oxidase-negative, rod-shaped bacteria with a prominent polysaccharide-based capsule.[3]
Klebsiella is named after German-Swiss microbiologist Edwin Klebs (1834–1913). Carl Friedlander described Klebsiella bacillus which is why it was termed Friedlander bacillus for many years. The species of Klebsiella are all gram-negative and usually non-motile. They tend to be shorter and thicker when compared to others in the family Enterobacteriaceae.
Klebsiella species are found everywhere in nature. This is thought to be due to distinct sublineages developing specific niche adaptations, with associated biochemical adaptations which make them better suited to a particular environment. They can be found in water, soil, plants, insects and other animals including humans,[4][5] including as part of the human and animal's normal flora in the nose, mouth and intestines.
List of species
[edit]- K. aerogenes, previously known as Enterobacter aerogenes and Bacterium aerogenes
- K. granulomatis
- K. oxytoca
- K. michiganensis
- K. pneumoniae (type-species)
- K. p. subsp. ozaenae
- K. p. subsp. pneumoniae
- K. p. subsp. rhinoscleromatis
- K. quasipneumoniae
- K. q. subsp. quasipneumoniae
- K. q. subsp. similipneumoniae
- K. grimontii
- K. variecola
Features
[edit]Klebsiella bacteria tend to be rounder and thicker than other members of the family Enterobacteriaceae. They typically occur as straight rods with rounded or slightly pointed ends. They can be found singly, in pairs, or in short chains. Diplobacillary forms are commonly found in vivo.[6]
They have no specific growth requirements and grow well on standard laboratory media, but grow best between 35 and 37 °C and at pH 7.2.[7] The species are facultative anaerobes, and most strains can survive with citrate and glucose as their sole carbon sources and ammonia as their sole nitrogen source.[6]
Members of the genus produce a prominent capsule, or slime layer, which can be used for serologic identification, but molecular serotyping may replace this method.[8]
Members of the genus Klebsiella typically express two types of antigens on their cell surfaces. The first, O antigen, is a component of the lipopolysaccharide (LPS), of which 9 varieties exist. The second is K antigen, a capsular polysaccharide with more than 80 varieties.[9] Both contribute to pathogenicity and form the basis for serogrouping. Based on those two major antigenic determinants several vaccines have been designed.[10]
In humans
[edit]Klebsiella species are routinely found in the human nose, mouth, and gastrointestinal tract as normal flora; however, they can also behave as opportunistic human pathogens.[6] Klebsiella species are known to also infect a variety of other animals, both as normal flora and opportunistic pathogens.[4]
Klebsiella organisms can lead to a wide range of disease states, notably pneumonia, urinary tract infections, sepsis, meningitis, diarrhea, peritonitis and soft tissue infections.[6][11] Klebsiella species have also been implicated in the pathogenesis of ankylosing spondylitis and other spondyloarthropathies.[12] The majority of human Klebsiella infections are caused by K. pneumoniae, followed by K. oxytoca. Infections are more common in the very young, very old, and those with other underlying diseases, such as cancer,[4] and most infections involve contamination of an invasive medical device.[6]
During the last 40 years, many trials for constructing effective K. pneumoniae vaccines have been tried,[13] and new techniques were followed to construct vaccines against Klebsiella.[14] However, currently, no Klebsiella vaccine has been licensed for use in the US. K. pneumoniae is the most common cause of nosocomial respiratory tract and premature intensive care infections, and the second-most frequent cause of Gram-negative bacteraemia and urinary tract infections . Drug-resistant isolates remain an important hospital-acquired bacterial pathogen, add significantly to hospital stays, and are especially problematic in high-impact medical areas such as intensive care units. This antimicrobial resistance is thought to be attributable mainly to multidrug efflux pumps.[15] The ability of K. pneumoniae to colonize the hospital environment, including carpeting, sinks, flowers, and various surfaces, as well as the skin of patients and hospital staff, has been identified as a major factor in the spread of hospital-acquired infections.[4]
In animals
[edit]In addition to certain Klebsiella spp. being discovered as human pathogens, others such as K. variicola have been identified as emerging pathogens in humans and animals alike. For instance, K. variicola has been identified as one of the causes of bovine mastitis.[16][17]
In plants
[edit]In plant systems, Klebsiella can be found in a variety of plant hosts. K. pneumoniae and K. oxytoca are able to fix atmospheric nitrogen into a form that can be used by plants, thus are called associative nitrogen fixers or diazotrophs.[5][18] The bacteria attach strongly to root hairs and less strongly to the surface of the zone of elongation and the root cap mucilage.[19] They are bacteria of interest in an agricultural context, due to their ability to increase crop yields under agricultural conditions.[20] Their high numbers in plants are thought to be at least partly attributable to their lack of a flagellum, as flagella are known to induce plant defenses.[21] Additionally, K. variicola is known to associate with a number of different plants including banana trees,[22] sugarcane[23] and has been isolated from the fungal gardens of leaf-cutter ants.[24]
See also
[edit]References
[edit]- ^ Trevisan, V. "Caratteri di alcuni nuovi generi di Batteriaceae [Characteristics of some new genera of Bacteriaceae]." Atti. Accad. Fis.-Med.-Stat. Milano (Ser 4) (1885) 3:92-106.
- ^ "Klebsiella". NCBI taxonomy. Bethesda, MD: National Center for Biotechnology Information. Retrieved 24 April 2019.
- ^ Ryan KJ; Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. p. 370. ISBN 978-0-8385-8529-0.
- ^ a b c d Bagley S (1985). "Habitat association of Klebsiella species". Infect Control. 6 (2): 52–8. doi:10.1017/S0195941700062603. PMID 3882590. S2CID 22799991.
- ^ a b Brisse S, Grimont F, Grimont PD (2006). Prokaryotes. New York, NY: Springer New York. pp. 159–196. ISBN 9783540325246.
- ^ a b c d e Ristuccia, Patricia A; Cunha Burke A (1984). "Klebsiella". Topics in Clinical Microbiology. 5 (7): 343–348. JSTOR 30144997.
- ^ Ristucci, Patricia; Cunha, Burke (July 1984). "Klebsiella". Infection Control. 5 (7): 343–348. doi:10.1017/S0195941700060549. JSTOR 30144997. PMID 6564087. S2CID 248999074.
- ^ Brisse, Sylvain; S Issenhuth-Jeanjean; P AD Grimont (2004). "Molecular Serotyping of Klebsiella Species Isolates by Restriction of the Amplified Capsular Antigen Gene Cluster". Journal of Clinical Microbiology. 42 (8): 3388–3398. doi:10.1128/jcm.42.8.3388-3398.2004. PMC 497587. PMID 15297473.
- ^ Podschun, R; Ullmann, U (October 1998). "Klebsiella spp. as Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors". Clinical Microbiology Reviews. 11 (4): 589–603. PMC 88898.
- ^ Ahmad, TA; El-Sayed, LH; Medhat, H; Hessen, A; El-Ashry, ESH (2012). "Development of immunization trials against Klebsiella pneumoniae". Vaccine. 30 (14): 2411–2420. doi:10.1016/j.vaccine.2011.11.027. PMID 22100884.
- ^ Podschun R, Ullmann U (1998). "Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors". Clin Microbiol Rev. 11 (4): 589–603. doi:10.1128/CMR.11.4.589. PMC 88898. PMID 9767057.
- ^ Sieper, Joachim; Braun, Jürgen (2011). Ankylosing Spondylitis in Clinical Practice. London: Springer-Verlag. p. 9. ISBN 978-0-85729-179-0.
- ^ Ahmad, TA; El-Sayed, LH; Haroun,M; Hussin, A; El-Ashry, ESH (2012). "Development of immunization trials against Klebsiella pneumoniae". Vaccine. 30 (14): 2411–2420. doi:10.1016/j.vaccine.2011.11.027. PMID 22100884.
- ^ Ahmad, TA; El-Sayed, LH; Haroun,M; Hussin, A; El-Ashry, ESH (2012). "Development of a new trend conjugate vaccine for the prevention of Klebsiella pneumoniae". Infectious Disease Reports. 4 (2): e33. doi:10.4081/idr.2012.e33. PMC 3892636. PMID 24470947.
- ^ Ogawa, Wakano; Li, Dai-Wei; Yu, Ping; Begum, Anowara; Mizushima, Tohru; Kuroda, Teruo; Tsuchiya, Tomofusa (2005). "Multidrug resistance in Klebsiella pneumoniae MGH78578 and cloning of genes responsible for the resistance". Biological & Pharmaceutical Bulletin. 28 (8): 1505–1508. doi:10.1248/bpb.28.1505. PMID 16079502.
- ^ Davidson, Fraser W.; Whitney, Hugh G.; Tahlan, Kapil (2015-10-29). "Genome Sequences of Klebsiella variicola Isolates from Dairy Animals with Bovine Mastitis from Newfoundland, Canada". Genome Announcements. 3 (5): e00938–15. doi:10.1128/genomeA.00938-15. ISSN 2169-8287. PMC 4566169. PMID 26358587.
- ^ Podder, Milka P.; Rogers, Laura; Daley, Peter K.; Keefe, Greg P.; Whitney, Hugh G.; Tahlan, Kapil (2014). "Klebsiella Species Associated with Bovine Mastitis in Newfoundland". PLOS ONE. 9 (9): e106518. Bibcode:2014PLoSO...9j6518P. doi:10.1371/journal.pone.0106518. PMC 4152263. PMID 25180510.
- ^ Cakmaki ML, Evans HJ, Seidler RJ (1981). "Characteristics of a nitrogen-fixing Klebsiella oxytoca isolated from wheat roots". Plant and Soil. 61 (1–2): 53–64. Bibcode:1981PlSoi..61...53C. doi:10.1007/BF02277362. S2CID 21625282.
- ^ Haahtela, K; Laakso T; Korhonen TK (1986). "Associative nitrogen fixation by Klebsiella spp.: Adhesion sites and inoculation effects on grass roots". Applied and Environmental Microbiology. 52 (5): 1074–1079. Bibcode:1986ApEnM..52.1074H. doi:10.1128/aem.52.5.1074-1079.1986. PMC 239175. PMID 16347205.
- ^ Riggs, PJ; Chelius MK; Iniguez AL; Kaeppler SM; Triplett EW (2001). "Enhanced maize productivity by inoculation with diazotrophic bacteria". Australian Journal of Plant Physiology. 28 (9): 829–836. doi:10.1071/PP01045.
- ^ Fouts, Derrick E.; Tyler, Heather L.; Deboy, Robert T.; Daugherty, Sean; Ren, Qinghu; Badger, Jonathan H.; Durkin, Anthony S.; Huot, Heather; Shrivastava, Susmita; Kothari, Sagar; Dodson, Robert J.; Mohamoud, Yasmin; Khouri, Hoda; Roesch, Luiz F. W.; Krogfelt, Karen A.; Struve, Carsten; Triplett, Eric W.; Methé, Barbara A. (2008). "Complete Genome Sequence of the N2-Fixing Broad Host Range Endophyte Klebsiella pneumoniae 342 and Virulence Predictions Verified in Mice". PLOS Genetics. 4 (7): e1000141. doi:10.1371/journal.pgen.1000141. PMC 2453333. PMID 18654632.

- ^ Rosenblueth, Mónica; Martínez, Lucía; Silva, Jesús; Martínez-Romero, Esperanza (2004-01-01). "Klebsiella variicola, A Novel Species with Clinical and Plant-Associated Isolates". Systematic and Applied Microbiology. 27 (1): 27–35. Bibcode:2004SyApM..27...27R. doi:10.1078/0723-2020-00261. PMID 15053318. S2CID 40606728.
- ^ Wei, Chun-Yan; Lin, Li; Luo, Li-Jing; Xing, Yong-Xiu; Hu, Chun-Jin; Yang, Li-Tao; Li, Yang-Rui; An, Qianli (2013-11-19). "Endophytic nitrogen-fixing Klebsiella variicola strain DX120E promotes sugarcane growth". Biology and Fertility of Soils. 50 (4): 657–666. doi:10.1007/s00374-013-0878-3. ISSN 0178-2762. S2CID 15594459.
- ^ Pinto-Tomás, Adrián A.; Anderson, Mark A.; Suen, Garret; Stevenson, David M.; Chu, Fiona S. T.; Cleland, W. Wallace; Weimer, Paul J.; Currie, Cameron R. (2009-11-20). "Symbiotic Nitrogen Fixation in the Fungus Gardens of Leaf-Cutter Ants". Science. 326 (5956): 1120–1123. Bibcode:2009Sci...326.1120P. doi:10.1126/science.1173036. ISSN 0036-8075. PMID 19965433. S2CID 3119587.
External links
[edit]- Klebsiella article from eMedicine.com


 French
French Deutsch
Deutsch