Markó–Lam deoxygenation
The Markó–Lam deoxygenation is an organic chemistry reaction where the hydroxy functional group in an organic compound is replaced by a hydrogen atom to give an alkyl group.[1][2] The Markó-Lam reaction is a variant of the Bouveault–Blanc reduction[3] and an alternative to the classical Barton–McCombie deoxygenation. It is named for the Belgian chemists István Markó and Kevin Lam.[4]
The main features of the reaction are:
- short reaction time (5 seconds to 5 minutes).
- the use of a stable toluate derivative.
- the use of SmI2/HMPA system or electrolysis instead of the classical and difficult to remove tributyltin hydride.

Mechanism
[edit]A hydroxyl group is first derivitised into a stable and very often crystalline toluate derivative. The aromatic ester is submitted to a monoelectronical reduction, by the use of SmI2/HMPA[5] or by electrolysis,[6] to yield the a radical-anion which decomposes into the corresponding carboxylate and into the radical of the alkyl fragment.

This radical could be used for further chemical reactions or can abstract a hydrogen atom to form the deoxygenated product.
Variations
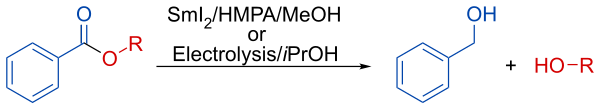
[edit]In presence of methanol or isopropanol, the reduction lead to the selective deprotection of the aromatic esters.[7]
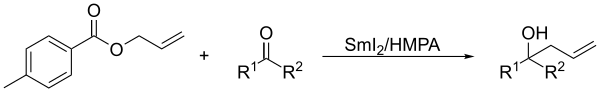
In presence of ketones, allylic derivatives lead to the coupling product when treated in Barbier's conditions with samarium diiodide.[8]
Scope
[edit]The Markó-Lam reaction was used as a final step in the total synthesis of Trifarienol B:[9]

References
[edit]- ^ "Alkane synthesis by deoxygenation". Organic-chemistry.org. Retrieved 2010-01-01.
- ^ マルコ・ラム脱酸素化 Marko-Lam Deoxygenation – ODOOS -合成反応データベース- by Chem-Station. Chem-station.com (2010-06-06). Retrieved on 2014-01-28.
- ^ Bouveault, L.; Blanc, G. L. (1904). "Transformation des acides monobasiques saturés dans les alcools primaires correspondants" [Transforming saturated monobasic acids into the corresponding primary alcohols]. Bull. Soc. Chim. Fr. (in French). 31: 666–672.
- ^ "Home". lamresearchgroup.com.
- ^ Lam, K.; Markó, I.E. (2008). "Using toluates as simple and versatile radical precursors". Org. Lett. 10 (13): 2773–2776. doi:10.1021/ol800944p. PMID 18507394.
- ^ Lam, K.; Markó, I.E. (2009). "Organic electrosynthesis using toluates as simple and versatile radical precursors" (PDF). Chem. Commun. 2009 (1): 95–97. doi:10.1039/b813545b. PMID 19082010.
- ^ Lam, K.; Markó, I.E. (2009). "Chemoselective chemical and electrochemical deprotections of aromatic esters". Org. Lett. 11 (13): 2752–2755. doi:10.1021/ol900828x. PMID 19492803.
- ^ Lam, K.; Markó, I.E. (2009). "Toluates: Unexpectedly versatile reagents". Tetrahedron. 65 (52): 10930–10940. doi:10.1016/j.tet.2009.09.111.
- ^ Takahashi, K.; Akao, R. & Honda, T. (2009). "Efficient diastereoselective synthesis of trifarane-type sesquiterpenes, trifarienols A and B". J. Org. Chem. 74 (9): 3424–3429. doi:10.1021/jo900369t. PMID 19334700.


 French
French Deutsch
Deutsch