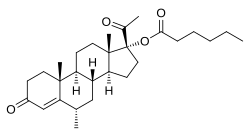
Medroxyprogesterone caproate
 | |
| Clinical data | |
|---|---|
| Other names | MPC; Medroxyprogesterone capronate; Medroxyprogesterone hexanoate; 6α-Methyl-17α-hydroxyprogesterone hexanoate; 6α-Methyl-17α-hydroxypregn-4-ene-3,20-dione hexanoate |
| Routes of administration | Intramuscular injection |
| Drug class | Progestogen; Progestin; Progestogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H42O4 |
| Molar mass | 442.640 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Medroxyprogesterone caproate (MPC) is a progestin and a progestogen ester which was synthesized in 1958 but was never marketed.[1][2] It has been confused with hydroxyprogesterone caproate (OHPC) and medroxyprogesterone acetate (MPA) in a number of publications.[3][4][5][6][7][8][9][10][11][12] In addition to MPA and OHPC, analogues of MPC include chlormadinone caproate, gestonorone caproate, megestrol caproate, and methenmadinone caproate.
See also
[edit]References
[edit]- ^ Babcock JC, Gutsell ES, Herr ME, Hogg JA, Stucki JC, Barnes LE, Dulin WE (1958). "6α-Methyl-17α-hydroxyprogesterone 17-acylates; a new class of potent progestins". Journal of the American Chemical Society. 80 (11): 2904–2905. Bibcode:1958JAChS..80.2904B. doi:10.1021/ja01544a079. ISSN 0002-7863.
- ^ Barton DH, Taylor WC (1958). "510. Photochemical transformations. Part IV. The photochemistry of prednisone acetate". Journal of the Chemical Society (Resumed): 2500–2510. doi:10.1039/jr9580002500. ISSN 0368-1769.
- ^ Pasqualini JR, Paris J, Sitruk-Ware R, Chetrite G, Botella J (April 1998). "Progestins and breast cancer". The Journal of Steroid Biochemistry and Molecular Biology. 65 (1–6): 225–235. doi:10.1016/S0960-0760(98)00028-4. PMID 9699877. S2CID 28416130.
- ^ Pasqualini JR, Ebert C (June 1999). "Biological effects of progestins in breast cancer". Gynecological Endocrinology. 13 (Suppl 4): 11–19. doi:10.1080/gye.13.s4.11.19. PMID 12227897.
- ^ Pasqualini JR, Chetrite GS (December 2010). "Biological responses of progestogen metabolites in normal and cancerous human breast". Hormone Molecular Biology and Clinical Investigation. 3 (3): 427–435. doi:10.1515/HMBCI.2010.066. PMID 25961215. S2CID 41680565.
- ^ Lantta M, Kahanpää K, Kärkkäinen J, Lehtovirta P, Wahlström T, Widholm O (June 1984). "Estradiol and progesterone receptors in two cases of endometrial stromal sarcoma". Gynecologic Oncology. 18 (2): 233–239. doi:10.1016/0090-8258(84)90031-3. PMID 6735266.
- ^ Gusberg SB, Shingleton HM, Deppe G (1988). Female genital cancer. Churchill Livingstone. p. 374. ISBN 978-0-443-08525-3.
- ^ Proceedings. American Cancer Society and National Cancer Institute of the U.S. Public Health Service, Federal Security Agency. 1970. p. 376.
- ^ Nichols DH, Evrard JR (1985). Ambulatory Gynecology. Harper & Row. p. 518. ISBN 978-0-06-141815-0.
- ^ Goodman LS, Gilman A (1996). Goodman & Gilman's the Pharmacological Basis of Therapeutics. McGraw-Hill, Health Professions Division. pp. 1427, 1823, 1858. ISBN 978-0-07-026266-9.
- ^ Endokrinologie. Johann Ambrosius Barth Verlag. 1969. p. 431.
- ^ McKinnon AO, Tarrida Del Marmol Figueroa S, Nobelius AM, Hyland JH, Vasey JR (1993). "Failure of medroxyprogesterone caproate to maintain pregnancy in ovariectomised mares". Equine Vet J. 25 (2): 158–160. doi:10.1111/j.2042-3306.1993.tb02928.x. PMID 8467776.


 French
French Deutsch
Deutsch