Niementowski quinoline synthesis
| Niementowski quinoline synthesis | |
|---|---|
| Named after | Stefan Niementowski |
| Reaction type | Ring forming reaction |
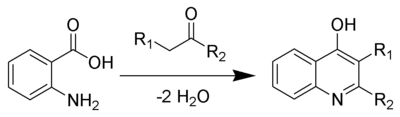
The Niementowski quinoline synthesis is the chemical reaction of anthranilic acids and ketones (or aldehydes) to form γ-hydroxyquinoline derivatives.[1][2][3][4]
Overview
[edit]In 1894, Niementowski reported that 2-phenyl-4-hydroxyquinoline was formed when anthranilic acid and acetophenone were heated to 120–130 °C. He later found that at higher heat, 200 °C, anthranilic acid and heptaldehyde formed minimal yields of 4-hydroxy-3-pentaquinoline.[5] Several reviews have been published.[6][7]
Variations
[edit]The temperatures required for this reaction make it less popular than other quinoline synthetic procedures. However, variations have been proposed to make this a more pragmatic and useful reaction. Adding phosphorus oxychloride to the reaction mixture mediates a condensation to make both isomers of an important precursor to an important α1-adrenoreceptor antagonist.[8] When the 3 position of an arylketone is substituted, it has been shown that a Niementowski-type reaction with propionic acid can produce a 4-hydroxyquinoline with 2-thiomethyl substitute.[9] The method has also been altered to occur with a catalytic amount of base,[10] or in the presence of polyphosphoric acid.[11]
Mechanism
[edit]Because of the similarity of these to the reagents in the Friedlander quinolone synthesis, a benzaldehyde with an aldehyde or ketone, the Niementowski quinoline synthesis mechanism is minimally different from that of the Friedländer synthesis. While studied in depth, two reaction pathways are possible and both have significant support.[5] The reaction is thought to begin with the formation of a Schiff base, and then proceed via an intra-molecular condensation to make an imine intermediate (see below). There is then a loss of water that leads to a ring closing and formation of the quinoline derivative. Most evidence supports this as the mechanism in normal conditions of 120–130 °C. Alternatively, the reaction begins with an intermolecular condensation and subsequent formation of the imine intermediate.[12] The latter has been shown to be more common under acidic or basic conditions. A similar pathway has been proposed for the Niementowski quinazoline synthesis.[13]
References
[edit]- ^ Niementowski, S. v. (1894). "Synthesen der Chinolinderivate". Chemische Berichte. 27 (2): 1394–1403. doi:10.1002/cber.18940270242.
- ^ Niementowski, S. v.; Orzechowski, B. (1895). "Synthesen der Chinolinderivate aus Anthranilsäure und Aldehyden". Chemische Berichte. 28 (3): 2809–2822. doi:10.1002/cber.18950280393.
- ^ Niementowski, S. v. (1905). "Ueber die Einwirkung des Benzoylessigesters auf Anthranilsäure (III. Mittheilung über Synthesen der Chinolinderivate)". Chemische Berichte. 38 (2): 2044–2051. doi:10.1002/cber.190503802142.
- ^ Niementowski, S. v. (1907). "Über die Einwirkung des Benzoylessigesters auf Anthranilsäure auf Anthrailsäure". Chemische Berichte. 40 (4): 4285–4294. doi:10.1002/cber.19070400444.
- ^ a b Hartz, pp. 376–384
- ^ Manske, R. H. (1942). "The Chemistry of Quinolines". Chem. Rev. 30: 127. doi:10.1021/cr60095a006.
- ^ Hisano, T. (1973). "Recent Studies on the Modified Niementowski 4-Quinazolone Synthesis. A Review". Org. Prep. Proced. Int. 5 (4): 145–193. doi:10.1080/00304947309355565.
- ^ Rosini, M.; Anontello, A.; Cavalli, A.; Bolognesi, M.; Minarini, A.; Marucci, G.; Poggesi, E.; Melchiorre, C. (2003). "Prazosin-Related Compounds. Effect of Transforming the Piperazinylquinazoline Moiety into an Aminomethyltetrahydroacridine System on the Affinity for α1-Adrenoreceptors". J. Med. Chem. 46 (23): 4895–4903. doi:10.1021/jm030952q. PMID 14584940.
- ^ Wang, M. -X., Liu, Y., Huang, Z, -T.; Liu; Huang (2001). "Novel and convenient synthesis of polyfunctionalized quinolines, quinolones and their annulation reactions". Tetrahedron Letters. 42 (13): 2553–2555. doi:10.1016/S0040-4039(01)00231-3.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chong, R. J.; Siddiqui, M. A.; Sneickus, V. (1983). "The synthesis of chiral annulet 1,4,7-triazacyclononanes". Tetrahedron Letters. 43 (21): 3795–3798. doi:10.1016/S0040-4039(02)00705-0.
- ^ Nahnda Kumar, R., Suresh, T., Mylithi, A., Mohan, P. S.; Suresh; Mythili; Mohan (2001). "A facile entry to pyrimido[4,5-b]quinolines and its thio analogues". Heterocycl. Commun. 7 (2): 193–198. doi:10.1515/HC.2001.7.2.193.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Marco-Contelles, José; PéRez-Mayoral, Elena; Samadi, Abdelouahid; Carreiras, María do Carmo; Soriano, Elena (2009). "Recent Advances in the Friedländer Reaction". Chemical Reviews. 109 (6): 2652–2671. doi:10.1021/cr800482c. PMID 19361199.
- ^ Hartz, pp. 440–453
Bibliography
[edit]- Hartz, R. (2011) in Name Reactions in Heterocyclic Chemistry II, Jie Jack Li, E. J. Corey (eds.), Wiley, ISBN 978-0-470-08508-0.


 French
French Deutsch
Deutsch