Pantetheine
 | |
 | |
| Names | |
|---|---|
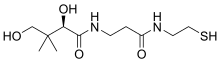
| Systematic IUPAC name (2R)-2,4-Dihydroxy-3,3-dimethyl-N-{3-oxo-3-[(2-sulfanylethyl)amino]propyl}butanamide | |
| Other names Pantetheine | |
| Identifiers | |
| |
3D model (JSmol) | |
| 3DMet | |
| 1714196 R | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.114 |
| EC Number |
|
| KEGG | |
| MeSH | Pantetheine |
PubChem CID | |
| UNII | |
| |
| |
| Properties | |
| C11H22N2O4S | |
| Molar mass | 278.37 g·mol−1 |
| Related compounds | |
Related compounds | Pantethine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Pantetheine is the cysteamine amide analog of pantothenic acid (vitamin B5). The dimer of this compound, pantethine is more commonly known, and is considered to be the most potent form of vitamin B5. Pantetheine is an intermediate in the catabolism of coenzyme A by the body.[1][2][3]
Metabolism
[edit]Pantetheine is the product of dephosphorylation of phosphopantetheine:
- phosphopantetheine → pantetheine + Pi
In E. coli, this reaction is catalyzed by for example alkaline phosphatase.[4] The reverse reaction, phosphopantetheine synthesis, is catalyzed by various kinases:[5]
- phosphopantetheine + ATP → pantetheine + ADP
These kinases are able to act upon pantothenoic acid as well and are present in both microorganisms and animal livers.[5]
Pantetheine is degraded by pantetheinase, which splits it into cysteamine and pantothenic acid:[3]
- pantetheine → cysteamine + pantothenate
Prebiotic evolution
[edit]Since pantetheine is a part of coenzyme A, a common cofactor, it is thought to have been present in prebiotic soup. A synthesis mechanism has also been suggested.[6]
References
[edit]- ^ Hoagland MB, Novelli GD (April 1954). "Biosynthesis of coenzyme A from phospho-pantetheine and of pantetheine from pantothenate". The Journal of Biological Chemistry. 207 (2): 767–773. doi:10.1016/S0021-9258(18)65696-0. PMID 13163064.
- ^ Cronan JE (June 2014). "The chain-flipping mechanism of ACP (acyl carrier protein)-dependent enzymes appears universal". The Biochemical Journal. 460 (2): 157–163. doi:10.1042/BJ20140239. PMID 24825445.
- ^ a b Nitto T, Onodera K (September 2013). "Linkage between coenzyme a metabolism and inflammation: roles of pantetheinase". Journal of Pharmacological Sciences. 123 (1): 1–8. doi:10.1254/jphs.13R01CP. PMID 23978960.
- ^ Jackowski S, Rock CO (April 1984). "Metabolism of 4'-phosphopantetheine in Escherichia coli". Journal of Bacteriology. 158 (1): 115–120. doi:10.1128/jb.158.1.115-120.1984. PMC 215387. PMID 6370952.
- ^ a b Brown GM (1970-01-01), Florkin M, Stotz EH (eds.), "Section d - Biosynthesis of Pantothenic Acid and Coenzyme A", Comprehensive Biochemistry, Metabolism of Vitamins and Trace Elements, vol. 21, Elsevier, pp. 73–80, doi:10.1016/b978-0-444-40871-6.50012-x, retrieved 2023-10-29
- ^ Keefe AD, Newton GL, Miller SL (February 1995). "A possible prebiotic synthesis of pantetheine, a precursor to coenzyme A". Nature. 373 (6516): 683–685. Bibcode:1995Natur.373..683K. doi:10.1038/373683a0. PMID 7854449. S2CID 4255864.


 French
French Deutsch
Deutsch