Phosphorus pentasulfide
 | |
 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.013.858 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| P4S10 | |
| Molar mass | 444.50 g/mol |
| Appearance | Yellow solid |
| Odor | Rotten eggs[1] |
| Density | 2.09 g/cm3 |
| Melting point | 288 °C (550 °F; 561 K) |
| Boiling point | 514 °C (957 °F; 787 K) |
| Hydrolyses | |
| Solubility in other solvents |
|
| Vapor pressure | 1 mmHg (300 °C)[1] |
| Structure | |
| triclinic, aP28 | |
| P1 (No. 2) | |
| Td | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 389 mg/kg (oral, rat)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) | TWA 1 mg/m3[1] |
REL (Recommended) | TWA 1 mg/m3 ST 3 mg/m3[1] |
IDLH (Immediate danger) | 250 mg/m3[1] |
| Related compounds | |
Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Phosphorus pentasulfide is the inorganic compound with the formula P2S5 (empirical) or P4S10 (molecular). This yellow solid is the one of two phosphorus sulfides of commercial value. Samples often appear greenish-gray due to impurities. It is soluble in carbon disulfide but reacts with many other solvents such as alcohols, DMSO, and DMF.[3]
Structure and synthesis
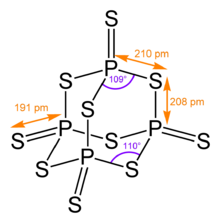
[edit]Its tetrahedral molecular structure is similar to that of adamantane and almost identical to the structure of phosphorus pentoxide.[4]
Phosphorus pentasulfide is obtained by the reaction of liquid white phosphorus (P4) with sulfur above 300 °C. The first synthesis of P4S10 by Berzelius in 1843[5] was by this method. Alternatively, P4S10 can be formed by reacting elemental sulfur or pyrite, FeS2, with ferrophosphorus, a crude form of Fe2P (a byproduct of white phosphorus (P4) production from phosphate rock):
- 4 Fe2P + 18 S → P4S10 + 8 FeS
- 4 Fe2P + 18 FeS2 P4S10 + 26 FeS
Applications
[edit]Approximately 150,000 tons of P4S10 are produced annually. The compound is mainly converted to other derivatives for use as lubrication additives such as zinc dithiophosphates. It is widely used in the production of sodium dithiophosphate for applications as a flotation agent in the concentration of molybdenite minerals. It is also used in the production of pesticides such as Parathion and Malathion.[6] It is also a component of some amorphous solid electrolytes (e.g. Li2S-P2S5) for some types of lithium batteries.
Phosphorus pentasulfide is a dual-use material, for the production of early insecticides such as Amiton and also for the manufacture of the related VX nerve agents.
Phosphorus pentasulfide reacts with ethanol to give diethyl dithiophosphoric acid:[7]
- P2S5 + 4 C2H5OH → 2 (C2H5O)2PS2H + H2S
diorganodithiophosphoric acids are used to produce metal dithiophosphates.
Reactivity
[edit]Due to hydrolysis by atmospheric moisture, P4S10 evolves hydrogen sulfide H2S, thus P4S10 is associated with a rotten egg odour. Aside from H2S, hydrolysis of P4S10 eventually gives phosphoric acid:
- P4S10 + 16 H2O → 4 H3PO4 + 10 H2S
Other mild nucleophiles react with P4S10, including alcohols and amines. Reaction with ammonium chloride gives the polymeric (SPN)∞.[8] Aromatic compounds such as anisole, ferrocene and 1-methoxynaphthalene react to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides such as Lawesson's reagent:
- P4S10 + 4 ArH → 2 [ArPS2]2 + 2 H2S (Ar = aryl)
P4S10 is used as a thionation reagent. Reactions of this type require refluxing solvents such as benzene, dioxane, or acetonitrile with P4S10 dissociating into P2S5. Some ketones, esters, and imides are converted to the corresponding thiocarbonyls. Amides give thioamides. With 1,4-diketones the reagent forms thiophenes. It is also used to deoxygenate sulfoxides. The use of P4S10 has been displaced by the aforementioned Lawesson's reagent.[9]
P4S10 reacts with pyridine to form the complex P2S5(pyridine)2.[10]
References
[edit]- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0510". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Phosphorus pentasulfide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Scott D. Edmondson, Mousumi Sannigrahi "Phosphorus(V) sulfide" Encyclopedia of Reagents for Organic Synthesis 2004 John Wiley & Sons. doi:10.1002/047084289X.rp166s.pub2
- ^ Corbridge, D. E. C. (1995). Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology (5th ed.). Amsterdam: Elsevier. ISBN 0-444-89307-5.
- ^ Berzelius, Jons J. (1843). "Ueber die Verbindungen des Phosphors mit Schwefel", parts I and II. Annalen der Chemie und Pharmacie, vol. 46, issue 2, pp. 129–154, 251–281. doi:10.1002/jlac.18430460202 and 10.1002/jlac.18430460303.
- ^ Bettermann, G.; Krause, W.; Riess, G.; Hofmann, T. (2002). "Phosphorus Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_527. ISBN 3527306730.
- ^ Coldbery, David E.; Fernelius, W. Conard; Shamma, Maurice (1960). "Chromium(III) O,O′-Diethyl Dithiophosphate". Inorganic Syntheses. 6: 142. doi:10.1002/9780470132371.ch44.
- ^ Almasi, Lucreţia (1971). "The Sulfur–Phosphorus Bond". In Senning, Alexander (ed.). Sulfur in Organic and Inorganic Chemistry. Vol. 1. New York: Marcel Dekker. p. 69. ISBN 0-8247-1615-9. LCCN 70-154612.
- ^ Ozturk, T.; Ertas, E.; Mert, O. (2010). "A Berzelius Reagent, Phosphorus Decasulfide (P4S10), in Organic Syntheses". Chemical Reviews. 110 (6): 3419–3478. doi:10.1021/cr900243d. PMID 20429553.
- ^ Bergman, Jan; Pettersson, Birgitta; Hasimbegovic, Vedran; Svensson, Per H. (2011). "Thionations Using a P4S10−Pyridine Complex in Solvents Such as Acetonitrile and Dimethyl Sulfone". The Journal of Organic Chemistry. 76 (6): 1546–1553. doi:10.1021/jo101865y. PMID 21341727.


 French
French Deutsch
Deutsch