Rhodium hexafluoride
 | |
| Names | |
|---|---|
| IUPAC name rhodium(VI) fluoride | |
| Other names rhodium hexafluoride | |
| Identifiers | |
3D model (JSmol) | |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| F6Rh | |
| Molar mass | 216.91 g/mol |
| Appearance | black crystalline solid[1] |
| Density | 3.71g/mL[2] |
| Melting point | ≈ 70 °C (158 °F; 343 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Rhodium hexafluoride, also rhodium(VI) fluoride, (RhF6) is the inorganic compound of rhodium and fluorine. A black volatile solid,[1] it is a highly reactive material which starts to slowly thermally decompose already at room temperature and a rare example of a rhodium(VI) compound. It is one of seventeen known binary hexafluorides.
Rhodium hexafluoride was discovered by American radiochemists in 1961, soon after the discovery of ruthenium hexafluoride.[3] It is prepared by reaction of rhodium metal with an excess of elemental fluorine:[4]
- Rh + 3 F2 → RhF6
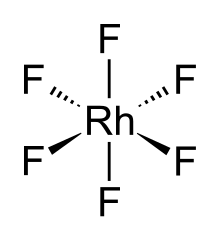
The RhF6 molecule has octahedral molecular geometry. Consistent with its d3 configuration, the six Rh–F bond lengths are equivalent, being 1.824 Å.[2] It crystallises in an orthorhombic space group Pnma with lattice parameters of a = 9.323 Å, b = 8.474 Å, and c = 4.910 Å.
Like some other metal fluorides, RhF6 is highly oxidizing. It attacks glass,[4] and can even react with elemental oxygen.[5]
References
[edit]- ^ a b c CRC Handbook of Chemistry and Physics, 90th Edition, CRC Press, Boca Raton, Florida, 2009, ISBN 978-1-4200-9084-0, Section 4, Physical Constants of Inorganic Compounds, p. 4-85.
- ^ a b Drews, T.; Supeł, J.; Hagenbach, A.; Seppelt, K. (2006). "Solid State Molecular Structures of Transition Metal Hexafluorides". Inorganic Chemistry. 45 (9): 3782–3788. doi:10.1021/ic052029f. PMID 16634614.
- ^ Chernick, Cedric L.; Claassen, Howard H.; Weinstock, Bernard (1961). "RHODIUM HEXAFLUORIDE". Journal of the American Chemical Society. 83 (14): 3165–3166. doi:10.1021/ja01475a046. ISSN 0002-7863.
- ^ a b 《无机化学丛书》第九卷:锰分族、铁系、铂系 (in Chinese). 北京: 科学出版社. 1991. p. 478. ISBN 7-03-002238-6.
- ^ Riedel, Sebastian; Kaupp, Martin (2009). "The highest oxidation states of the transition metal elements" (PDF). Coordination Chemistry Reviews. 253 (5–6). Elsevier: 606–624. doi:10.1016/j.ccr.2008.07.014.[permanent dead link]
Further reading
[edit]- Gmelins Handbuch der anorganischen Chemie, System Nr. 63, Rhodium, Part B1, pp. 266–268.
External links
[edit] Media related to Rhodium hexafluoride at Wikimedia Commons
Media related to Rhodium hexafluoride at Wikimedia Commons- Rhodium hexafluoride at webelements.com.


 French
French Deutsch
Deutsch