SCH-50911
 | |
| Names | |
|---|---|
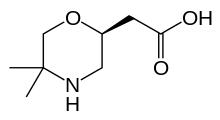
| Preferred IUPAC name 2-[(2S)-5,5-Dimethylmorpholin-2-yl]acetic acid | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| MeSH | SCH-50911 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C8H15NO3 | |
| Molar mass | 173.21 g·mol−1 |
| Melting point | 154.5 to 157 °C (310.1 to 314.6 °F; 427.6 to 430.1 K) (hydrochloride) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
SCH-50911 is a selective GABAB antagonist.[1] Its main applications are in pharmacology research.
SCH-50911 also acts as an anticonvulsant under normal conditions. SCH-50911 induces acute withdrawal syndrome in GHB-dependent rats, similar to the delirium tremens seen in human alcohol withdrawal, and can precipitate convulsions in GHB-dependent animals.[2]
References
[edit]- ^ Blythin DJ, Kuo SC, Shue HJ, McPhail AT, Chapman RW, Kreutner W, et al. (July 1996). "Substituted morpholine-2S-acetic acid derivatives: Sch 50911 and related compounds as novel GABAB antagonists". Bioorganic & Medicinal Chemistry Letters. 6 (13): 1529–34. doi:10.1016/S0960-894X(96)00267-3.
- ^ Quang LS, Colombo G, Lobina C, Maccioni P, Orru A, Gessa GL, et al. (August 2006). "Evaluation for the withdrawal syndrome from gamma-hydroxybutyric acid (GHB), gamma-butyrolactone (GBL), and 1,4-butanediol (1,4-BD) in different rat lines". Annals of the New York Academy of Sciences. 1074 (1): 545–58. Bibcode:2006NYASA1074..545Q. doi:10.1196/annals.1369.055. PMID 17105952. S2CID 86383425.


 French
French Deutsch
Deutsch