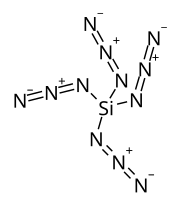
Silicon tetraazide
| Names | |
|---|---|
| Other names Tetraazidosilane | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| Si(N3)4 | |
| Molar mass | 196.1659 g/mol |
| Appearance | White crystals |
| Melting point | 212 °C (414 °F; 485 K) |
| Reacts | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Silicon tetraazide is a thermally unstable binary compound of silicon and nitrogen with a nitrogen content of 85.7% (by molar mass). This high-energy compound combusts spontaneously and can only be studied in a solution.[1][2][3] A further coordination to a six-fold coordinated structure such as a hexaazidosilicate ion [Si(N3)6]2−[4] or as an adduct with bidentate ligands Si(N3)4·L2[2] will result in relatively stable, crystalline solids that can be handled at room temperature.
Preparation
[edit]Silicon tetraazide is synthesized by conversion of silicon tetrachloride with sodium azide in benzene.[1][3]
The reaction of silicon tetrachloride with an excess of sodium azide at room temperature in acetonitrile will result in the formation of sodium hexaazidosilicate (Na2[Si(N3)6]) which by adding ligands such as 2,2′-bipyridine and 1,10-phenanthroline will result in stable silicon tetraazide adducts.[2] Other bases such as pyridine and tetramethylethylenediamine will not react with the hexaazidosilicate ion.[2]
Another preparation of a bis(triphenylphosphine)iminium hexaazidosilicate salt [(Ph3P)2N]2[Si(N3)6] is possible by conversion of bis(triphenylphosphine)iminium azide [(Ph3P)2N]N3 with silicon tetrachloride in acetonitrile, where Ph is phenyl.[4]
Properties
[edit]Silicon tetraazide is a white crystalline compound that will detonate at even 0 °C.[1] The pure compound, and also silicon chloride triazide SiCl(N3)3 and silicon dichloride diazide SiCl2(N3)2 contaminated samples, can detonate spontaneously without clear cause.[5] The compound is susceptible to hydrolysis.[3] It is soluble in diethylether and benzene.[1]
The addition compound with 2,2′-bipyridine is much more stable. A melting point of 212 °C with a melting enthalpy of 110 J/g is recorded. The DSC measurement shows at 265 °C a sharp exothermic reaction with an enthalpy of −2400 J/g. Similar results are found for the addition compound with 1,10-phenanthroline. As the hemiacetonitrile solvatated isolated compound expels solvent at 100 °C, and shows then in the DSC measurement from 240 °C onwards a strong exothermic reaction with a generated heat of 2300 J/g.[2] The enthalpies are higher than that of sodium azide with −800 J/g,[6] but still lower than the values encountered with classic explosives such as RDX with −4500 J/g.[2] The addition compounds are stable in solution. It can be concluded from IR-spectroscopy and proton NMR data that no dissociation occurs in silicon tetraazide and 2,2'-bipyridine or for example 1,10-phenanthroline.[2] The bis(triphenylphosphino)iminium hexaazidosilicate salt [(Ph3P)2N]2[Si(N3)6] on the other hand is relatively stable. The compound melts at 214 °C and shows in the DSC measurement at 250 °C a reaction.[4] One mass spectrometry coupled thermogravimetric analysis investigation indicated as reaction products nitrogen, silicon tetraazide and hydrazoic acid.[4]
Applications
[edit]A practical application of free silicon tetraazide is unlikely due to the high instability. In solution the compound has potential uses as raw material for nitrogen-rich materials.[2] One application as reagent in the manufacture of polyolefins has been patented.[7] The stabilized adducts can serve as energetic compounds as a replacement for lead azide.[2]
References
[edit]- ^ a b c d Wilberg, E.; Michaud, H.: Z. Naturforsch. B 9 (1954) S. 500.
- ^ a b c d e f g h i Portius, Peter; Filippou, Alexander C.; Schnakenburg, Gregor; Davis, Martin; Wehrstedt, Klaus-Dieter (2010). "Neutrale Lewis-Basen-Addukte des Siliciumtetraazids". Angewandte Chemie. 122 (43): 8185–8189. Bibcode:2010AngCh.122.8185P. doi:10.1002/ange.201001826.
- ^ a b c Gmelins Handbook of Inorganic Chemistry, 8th Edition, Silicon Supplement Volume B4, Springer-Verlag 1989, S. 46.
- ^ a b c d Filippou, Alexander C.; Portius, Peter; Schnakenburg, Gregor (2002). "The Hexaazidosilicate(IV) Ion: Synthesis, Properties, and Molecular Structure". Journal of the American Chemical Society. 124 (42): 12396–12397. doi:10.1021/ja0273187. PMID 12381165.
- ^ Bretherick's Handbook of Reactive Chemical Hazards, 7th revised edition, Academic Press 2006, ISBN 978-0-12-372563-9
- ^ T. Grewer: Thermal Hazards of Chemical Reactions, Industrial Safety Series 4, Elsevier 1994.
- ^ Nomura, M.; Tomomatsu, R.; Shimazaki, T.: EP 206 034 (1985) pdf-Download


 French
French Deutsch
Deutsch