Stacking (chemistry)
In chemistry, stacking refers to superposition of molecules or atomic sheets owing to attractive interactions between these molecules or sheets.
Metal dichalcogenide compounds
[edit]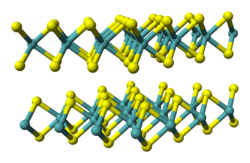
Metal dichalcogenides have the formula ME2, where M = a transition metal and E = S, Se, Te.[1] In terms of their electronic structures, these compounds are usually viewed as derivatives of M4+. They adopt stacked structures, which is relevant to their ability to undergo intercalation, e.g. by lithium, and their lubricating properties. The corresponding diselenides and even ditellurides are known, e.g., TiSe2, MoSe2, and WSe2.
Charge transfer salts
[edit]

A combination of tetracyanoquinodimethane (TCNQ) and tetrathiafulvalene (TTF) forms a strong charge-transfer complex referred to as TTF-TCNQ.[3] The solid shows almost metallic electrical conductance. In a TTF-TCNQ crystal, TTF and TCNQ molecules are arranged independently in separate parallel-aligned stacks, and an electron transfer occurs from donor (TTF) to acceptor (TCNQ) stacks.[4]
Graphite
[edit]
Graphite consists of stacked sheets of covalently bonded carbon.[5][6] The individual layers are called graphene. In each layer, each carbon atom is bonded to three other atoms forming a continuous layer of sp2 bonded carbon hexagons, like a honeycomb lattice with a bond length of 0.142 nm, and the distance between planes is 0.335 nm.[7] Bonding between layers is relatively weak van der Waals bonds, which allows the graphene-like layers to be easily separated and to glide past each other.[8] Electrical conductivity perpendicular to the layers is consequently about 1000 times lower.[9]
Linear chain compounds
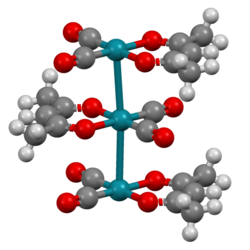
[edit]Linear chain compounds are materials composed of stacked arrays of metal-metal bonded molecules or ions. Such materials exhibit anisotropic electrical conductivity.[10] One example is Rh(acac)(CO)2 (acac = acetylacetonate, which stack with Rh···Rh distances of about 326 pm.[11] Classic examples include Krogmann's salt and Magnus's green salt.
Counterexample: benzene dimer and related species
[edit]π–π stacking is a noncovalent interaction between the pi bonds of aromatic rings.[12] Such "sandwich interactions" are however generally electrostatically repulsive. What is more commonly observed are either a staggered stacking (parallel displaced) or pi-teeing (perpendicular T-shaped) interaction both of which are electrostatic attractive.[13] For example, the most commonly observed interactions between aromatic rings of amino acid residues in proteins is a staggered stacked followed by a perpendicular orientation. Sandwiched orientations are relatively rare.[14] Pi stacking is repulsive as it places carbon atoms with partial negative charges from one ring on top of other partial negatively charged carbon atoms from the second ring and hydrogen atoms with partial positive charges on top of other hydrogen atoms that likewise carry partial positive charges.[15]

π–π interactions play a role in supramolecular chemistry, specifically the synthesis of catenane. The major challenge for the synthesis of catenane is to interlock molecules in a controlled fashion. Attractive π–π interactions exist between electron-rich benzene derivatives and electron-poor pyridinium rings.[16] [2]Catanene was synthesized by treating bis(pyridinium) (A), bisparaphenylene-34-crown-10 (B), and 1, 4-bis(bromomethyl)benzene (C) (Fig. 2). The π–π interaction between A and B directed the formation of an interlocked template intermediate that was further cyclized by substitution reaction with compound C to generate the [2]catenane product.
See also
[edit]- Noncovalent interaction
- Dispersion (chemistry)
- Cation–pi interaction
- Intercalation (biochemistry)
- Intercalation (chemistry)
References
[edit]- ^ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ D. Chasseau; G. Comberton; J. Gaultier; C. Hauw (1978). "Réexamen de la structure du complexe hexaméthylène-tétrathiafulvalène-tétracyanoquinodiméthane". Acta Crystallographica Section B. 34 (2): 689. Bibcode:1978AcCrB..34..689C. doi:10.1107/S0567740878003830.
- ^ P. W. Anderson; P. A. Lee; M. Saitoh (1973). "Remarks on giant conductivity in TTF-TCNQ". Solid State Communications. 13 (5): 595–598. Bibcode:1973SSCom..13..595A. doi:10.1016/S0038-1098(73)80020-1.
- ^ Van De Wouw, Heidi L.; Chamorro, Juan; Quintero, Michael; Klausen, Rebekka S. (2015). "Opposites Attract: Organic Charge Transfer Salts". Journal of Chemical Education. 92 (12): 2134–2139. Bibcode:2015JChEd..92.2134V. doi:10.1021/acs.jchemed.5b00340.
- ^ Delhaes, Pierre (2000). "Polymorphism of carbon". In Delhaes, Pierre (ed.). Graphite and precursors. Gordon & Breach. pp. 1–24. ISBN 9789056992286.
- ^ Pierson, Hugh O. (2012). Handbook of carbon, graphite, diamond, and fullerenes : properties, processing, and applications. Noyes Publications. pp. 40–41. ISBN 9780815517399.
- ^ Delhaes, P. (2001). Graphite and Precursors. CRC Press. ISBN 978-90-5699-228-6.
- ^ Chung, D. D. L. (2002). "Review Graphite". Journal of Materials Science. 37 (8): 1475–1489. doi:10.1023/A:1014915307738. S2CID 189839788.
- ^ Pierson, Hugh O. (1993). Handbook of carbon, graphite, diamond, and fullerenes : properties, processing, and applications. Park Ridge, N.J.: Noyes Publications. ISBN 0-8155-1739-4. OCLC 49708274.
- ^ Bera, J. K.; Dunbar, K. R. (2002). "Chain Compounds Based on Transition Metal Backbones: New Life for an Old Topic". Angew. Chem. Int. Ed. 41 (23): 4453–4457. doi:10.1002/1521-3773(20021202)41:23<4453::AID-ANIE4453>3.0.CO;2-1. PMID 12458505.
- ^ Huq, Fazlul; Skapski, Andrzej C. (1974). "Refinement of the crystal structure of acetylacetonatodicarbonylrhodium(I)". J. Cryst. Mol. Struct. 4 (6): 411–418. doi:10.1007/BF01220097. S2CID 96977904.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 114, ISBN 978-0-471-72091-1
- ^ Lewis M, Bagwill C, Hardebeck L, Wireduaah S (2016). "Modern Computational Approaches to Understanding Interactions of Aromatics". In Johnson DW, Hof F (eds.). Aromatic Interactions: Frontiers in Knowledge and Application. England: Royal Society of Chemistry. pp. 1–17. ISBN 978-1-78262-662-6.
- ^ McGaughey GB, Gagné M, Rappé AK (June 1998). "pi-Stacking interactions. Alive and well in proteins". The Journal of Biological Chemistry. 273 (25): 15458–63. doi:10.1074/jbc.273.25.15458. PMID 9624131.
- ^ Martinez CR, Iverson BL (2012). "Rethinking the term "pi-stacking"". Chemical Science. 3 (7): 2191. doi:10.1039/c2sc20045g. hdl:2152/41033. ISSN 2041-6520. S2CID 95789541.
- ^ Ashton PR, Goodnow TT, Kaifer AE, Reddington MV, Slawin AM, Spencer N, et al. (1989). "A [2] Catenane Made to Order". J. Angew. Chem. Int. Ed. 28 (10): 1396–1399. doi:10.1002/anie.198913961.
External links
[edit]- Luo R, Gilson HS, Potter MJ, Gilson MK (January 2001). "The physical basis of nucleic acid base stacking in water". Biophysical Journal. 80 (1): 140–148. Bibcode:2001BpJ....80..140L. doi:10.1016/S0006-3495(01)76001-8. PMC 1301220. PMID 11159389.
- Larry Wolf (2011): π-π (π-Stacking) interactions: origin and modulation


 French
French Deutsch
Deutsch